1.2 The periodic table
1.2.1 The periodic table
- The elements in the periodic table are arranged in order of atomic (proton) number and so
that elements with similar properties are in columns, known as groups.
- The table is called a periodic table because similar properties occur at regular intervals.
- Elements in the same group in the periodic table have the same number of electrons in their outer
shell (outer electrons) and this gives them similar chemical properties.
- A periodic table is available at ptable.com.
Position in the Periodic Table and Electron Arrangement
The periodic table is arranged based on the atomic number — the number of protons in the nucleus (and also the number of electrons in a neutral atom).
- Periods (Rows): Elements in the same period have the same number of electron
shells.
Example: Sodium (Na) and Magnesium (Mg) are in Period 3, so both have 3 electron shells. - Groups (Columns): Elements in the same group have the same number of valence
electrons.
Example:- Group 1 (e.g. Li, Na, K): all have 1 valence electron.
- Group 17 (e.g. F, Cl, Br): all have 7 valence electrons.
Atomic Number and Electron Configuration
The atomic number tells us how many electrons the atom has. These electrons fill up energy levels (shells) in a specific order (e.g., 2, 8, 8...). This helps us predict the chemical behaviour of an element.
Predicting Reactions and Reactivity
The number of valence electrons (from the group number) helps predict how an element will react:
- Group 1 – Alkali Metals:
- 1 valence electron → easily lost to form +1 ions.
- Very reactive, especially with water.
- Reactivity increases down the group.
- Group 7 – Halogens:
- 7 valence electrons → easily gain 1 electron to form –1 ions.
- Very reactive non-metals.
- Reactivity decreases down the group.
- Group 8 – Noble Gases:
- Full outer shell → very stable and unreactive.
1.2.2 Development of the Periodic Table
mostly taken from spec
- Before the discovery of protons, neutrons, and electrons, scientists tried to classify elements
based on their atomic weights.
- This led to periodic tables being incomplete and some elements were placed in inappropriate groups
if the strict order of atomic weights was followed.
Early Periodic Tables
Dalton's Periodic Table (by atomic weight)
 File from the Wellcome Collection. Licensed under CC-BY 4.0.
File from the Wellcome Collection. Licensed under CC-BY 4.0. Newlands' Law of Octaves
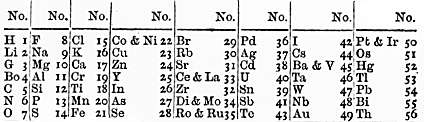
This is similar to how the periodic table is arranged today, and is an early version of how outer shell electrons contribute to groups in the periodic table.
Mendeleev's Period Table
- Mendeleev's period table overcame these problems by leaving gaps for elements that he thought had
not been discovered and ins oem places changed the order even when it did not conform to atomic weights.
- Elements with properties, able to be predicted by his table, were discovered and filled the gaps. Knowledge
of isotopes made it possible to explain why the order based on atomic weights was not always correct.
An early version of Mendeleev's Period Table
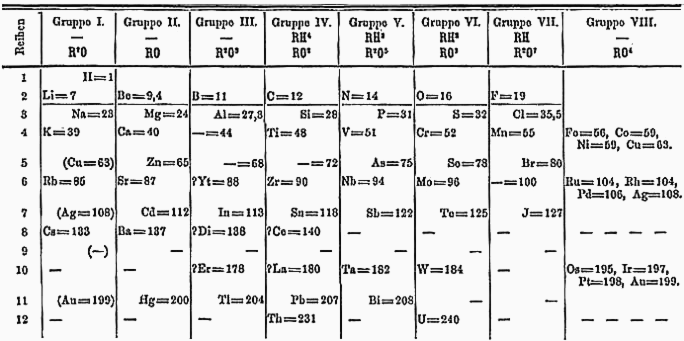
How this table works is (essentially):
Iodine has a lower atomic weight compared to tellurium, which means it should be
positioned before tellurium in Mendeleev's periodic table. However, iodine shares similar
chemical properties with chlorine and bromine. To align iodine with these elements in his table,
Mendeleev exchanged the places of iodine and tellurium.
1.2.3 Metals and non-metals
(stolen from spec)
- Elements that react to form positive ions are metals (e.g. Aluminium forms Al3+, as it
loses three electrons to form an ion.).
- Elements that do not form positive ions are non-metals (e.g. Oxygen forms O2-, as it gains
two electrons to form an ion.).
- The majority of elements are metals. Metals are found to the left
and towards the bottom of the periodic table. Non-metals are found
towards the right and top of the periodic table.
- Metalloids display both metallic and non-metallic properties.
Properties of metals vs non-metals
| Property | Metals | Non-Metals |
|---|---|---|
| Appearance | Shiny | Dull |
| State at Room Temperature | Solid (except mercury) | Solid, liquid, or gas |
| Density | High | Low |
| Melting and Boiling Points | High | Low (generally) |
| Electrical Conductivity | Good conductor | Poor conductor (except graphite) |
| Thermal Conductivity | Good | Poor |
| Malleability | Malleable | Brittle (if solid) |
| Ductility | Ductile | Not ductile |
| Type of Oxide Formed | Basic (/Alkaline) | Acidic |
| Reactivity with Acids | React to produce hydrogen | Generally no reaction |
1.2.4 Group 0
- The elements in group 0 are called the noble gases.
- They are unreactive and do not easily form molecules
because their atoms have stable arrangements of electrons (there are eight electrons
in their outer shell, which means it is "full", except helium which has only two electrons).
- The boiling points of the noble gases increase with increasing relative atomic mass (going down the group):
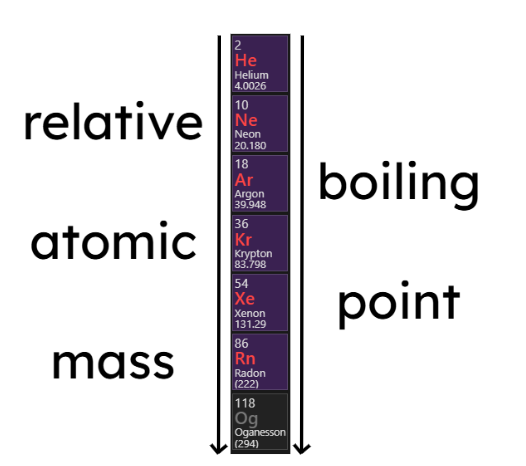
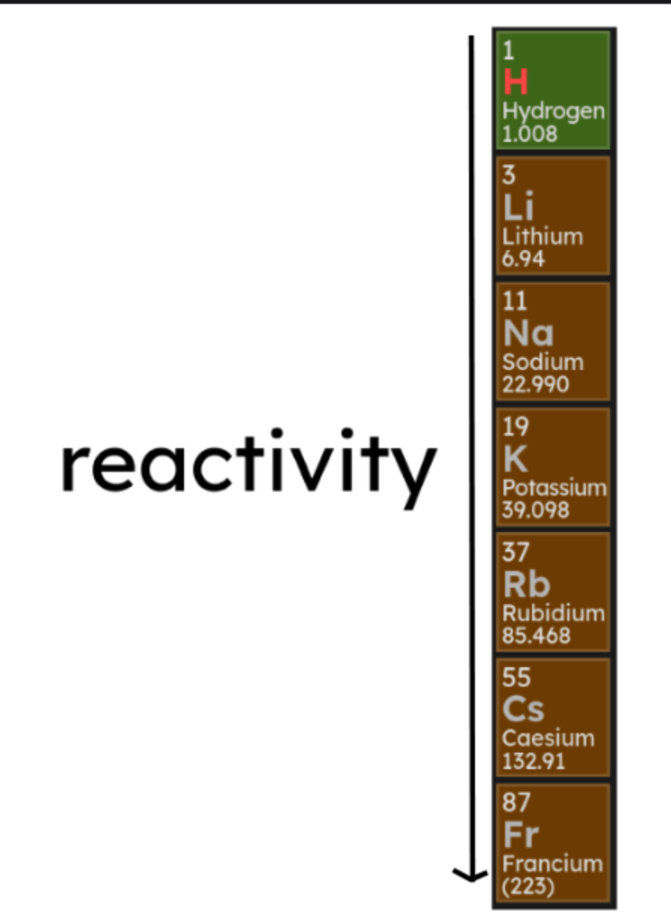
1.2.5 Group 1
- The elements in Group 1 of the periodic table are known as the
alkali metals.
- They have one electron in their outer shell and are highly reactive.
- They can form ionic bonds with non-metals in the form of positive ions.
- Their reactivity increases down the group:
Reactions of the first three elements with oxygen, chlorine and water
- The first three alkali metals (lithium, sodium, and potassium) react with oxygen, chlorine, and water in different ways. The table below summarises these reactions:
| Element | Reaction with Oxygen | Reaction with Chlorine | Reaction with Water |
|---|---|---|---|
| Lithium (Li) | Burns with a red flame to form white lithium oxide (Li2O). | Forms white lithium chloride (LiCl). | Fizzes slowly, producing hydrogen gas and lithium hydroxide (LiOH). |
| Sodium (Na) | Burns with a yellow-orange flame to form a mixture of sodium oxide (Na2O) and sodium peroxide (Na2O2). | Forms white sodium chloride (NaCl). | Fizzes more vigorously, melts into a ball, produces hydrogen and sodium hydroxide (NaOH). |
| Potassium (K) | Burns with a lilac flame to form potassium peroxide (K2O2). | Forms white potassium chloride (KCl). | Reacts very vigorously, ignites the hydrogen, forming potassium hydroxide (KOH). |
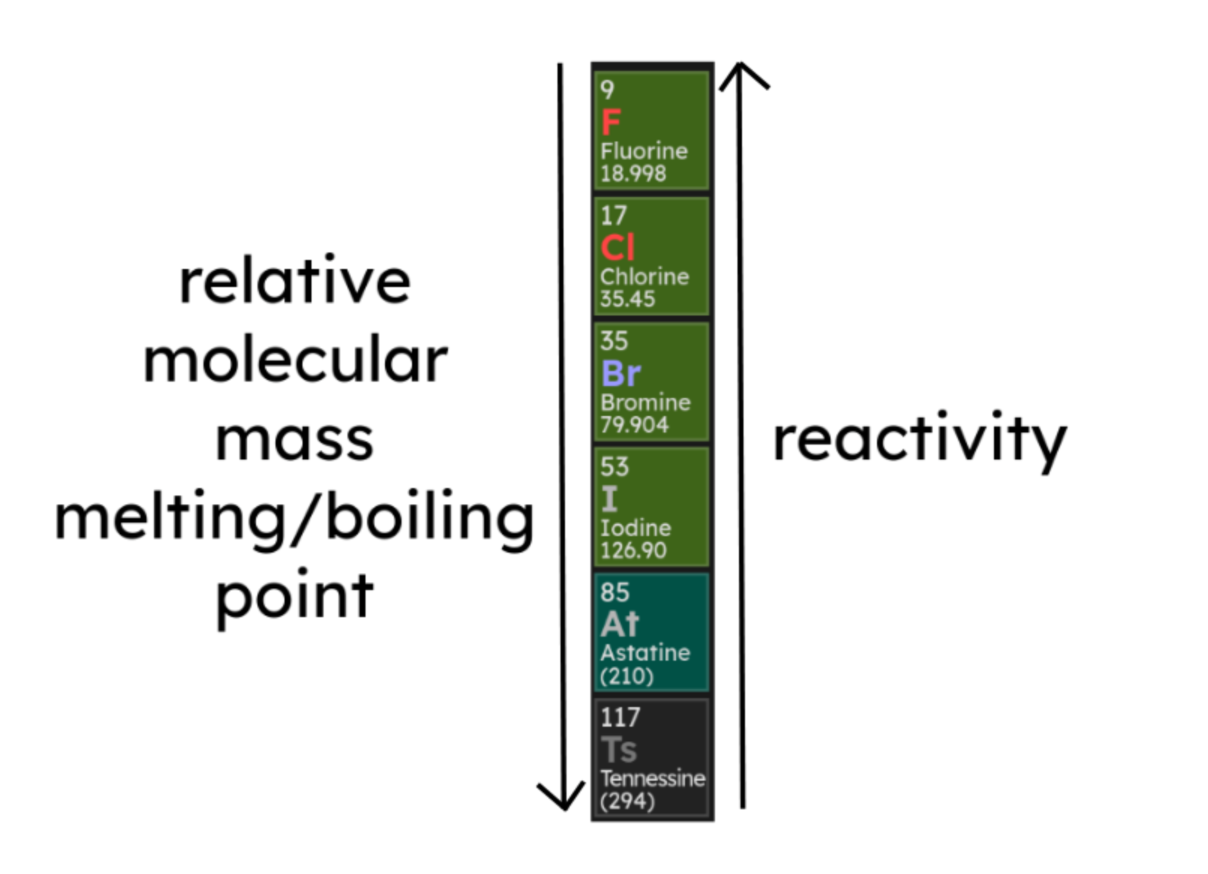
1.2.6 Group 7
- The elements in Group 7 of the periodic table are known as the
halogens and have similar reactions because they all have seven
electrons in their outer shell.
- They therefore form negative ions and form ionic bonds with metals.
- The halogens are non-metals and consist of molecules made of pairs of atoms,
for example Cl2.
- In Group 7, relative molecular mass, melting point and boiling point increase
going down the group, however the reactivity of the elements decreases:
Displacement
- A more reactive halogen can displace a less reactive halogen from
an aqueous solution of its salt. For example:
Cl2 (chlorine) + 2KBr (potassium bromide) → 2KCl (potassium chloride) + Br2 (bromine)
- Here, chlorine displaces bromine because it is more reactive.
Compounds formed with metals and non-metals
- When halogens react with metals, they form ionic compounds. The halogen atoms gain one electron to form negative halide ions (e.g., Cl-, Br-, I-).
Example: Sodium + Chlorine → Sodium chloride (NaCl; made up of Na+ and Cl-)
- When halogens react with non-metals, they form covalent compounds. The atoms share electrons to complete their outer shells.
Example: Hydrogen + Chlorine → Hydrogen chloride (HCl)
