10.1 Using the Earth's resources and obtaining potable water
10.1.1 Using the Earth's resources and sustainable development
- Humans can use the Earth's resources to provide warmthm, shelter, food and transport.
- These natural resources are supplemented by agriculture to provide food, timber, clothing, and fuels.
- Finite resources are resources where there is only a limited supply. Finite resources from the Earth, oceans,
and atmosphere, are processed to provide energy and materials.
- Finite resources include fossil fuels (oil, coal, and natural gas) and metal ores.
- Renewable resources are resources that can be replaced at the same rate as, or faster than, they are used.
- Examples of renewable resources include timber (if managed correctly), water, and wind energy.
- Sustainable resource management is using resources while leaving enough for future generations to meet their needs.
- Carbon-neutral resources come from plants. The carbon dioxide absorbed by the plants as they grow is equal to the carbon dioxide released when they are burned.
10.1.2 Potable water
- Potable water is water that is safe to drink.
- Fresh water is water that has a low concentration of dissolved salts and microbes.
- Most fresh water is found in lakes, rivers, and groundwater.
- In the UK, fresh water from rivers and lakes is treated to make it potable by:
- choosing an appropriate source of fresh water
- passing the water through filter beds (sand, gravel, and charcoal)
- sterilisation (using chlorine, ozone, or ultraviolet light) to kill microbes
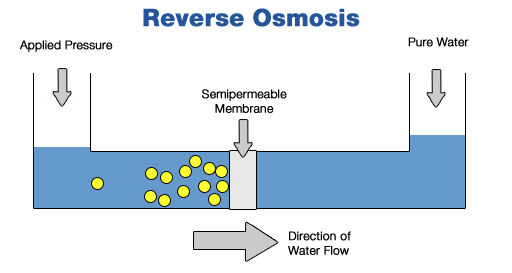
- Desalination methods include distillation (heating the water until it evaporates, then condensing the vapour) and reverse osmosis (forcing the water through a membrane that only allows water molecules to pass through).
- Both methods of desalination require large amounts of energy, making them expensive.
| Description | Name | Contains microbes | Contains dissolved substances |
|---|---|---|---|
| Water in the seas and oceans | Salt water | Yes | Yes - high concentration |
| Water in underground streams and rocks | Ground water | Yes | Low concentration |
| Water in underground streams and rocks, rivers, lakes, ice caps, and glaciers | Fresh water | Yes | Low concentration |
| Used water from homes, industry, and agriculture | Waste water | Yes | High concentration (not as high as sea water) |
| Water that is safe to drink | Potable water | A small number | Very low concentration |
| Water that is 100% water molecules | Pure water | No | No dissolved substances |
Distillation desalination diagram
 source
source Reverse osmosis diagram
 source
source Required Practical 8: analysis and purification of water samples from different sources, including pH, dissolved solids and distillation
Required practical: analysis and purification of water samples
- Sampling
- Collect labelled samples from each source (tap, river, groundwater, seawater, wastewater). Record location, time, temperature.
- Use clean glassware; avoid cross-contamination; store cold and test promptly.
- pH measurement
- Calibrate pH meter with pH 4.00 and 7.00 (and 10.00 if necessary). Rinse probe between samples.
- Measure and record pH and temperature for each sample; optionally compare with universal indicator paper/solution.
- Notes: pH affects solubility and disinfection effectiveness.
- Dissolved solids (TDS) / total dissolved solids
- Method A: Conductivity/TDS meter: calibrate, measure conductivity and convert to TDS using device factor; quick non-destructive test.
- Method B: Gravimetric (precise): filter sample to remove suspended solids, evaporate a known volume to dryness in a pre-weighed crucible (dry at ~105 °C), cool in desiccator, weigh residue. Calculate mg L⁻¹ = (mass residue / volume sample in L) × 1000.
- Include blanks and repeat measurements; account for hygroscopic salts and volatile losses as uncertainty sources.
- Additional tests (optional)
- Chloride: titration (AgNO3) or ion-selective electrode.
- Turbidity: nephelometer or visual comparison.
- Microbial presence: culture tests (if required) or use membrane filtration methods.
- Purification methods
- Pre-treatment: coarse filtration/sedimentation to remove suspended solids.
- Filtration: sand/gravel/activated charcoal for organics and particulates.
- Sterilisation: boiling, chemical (chlorination), UV or ozone to kill microbes.
- Desalination/distillation: simple distillation apparatus — heat sample, collect condensate; distilled water should have very low conductivity and low residue on evaporation.
- Reverse osmosis note: high energy, uses semipermeable membrane (lab demo usually limited to simple distillation).
- Distillation practical notes
- Set up round-bottom flask, condenser, and receiver; use boiling chips; control heating to avoid bumping.
- Collect fractions if required; measure pH, conductivity, and TDS of distillate and compare with feed.
- Record mass/volume of distillate and residue; calculate % removal of dissolved solids.
- Calculations and evaluation
- Calculate mg L⁻¹ dissolved solids, % removal after purification, and uncertainty (repeatability, instrument calibration, mass balance errors).
- Discuss limitations: energy cost of distillation, incomplete removal of volatile contaminants, adsorption capacity of charcoal, sampling errors.
- Safety: handle hot apparatus, corrosive reagents, and contaminated samples with appropriate PPE and waste disposal.
10.1.3 Waste Water Treatment
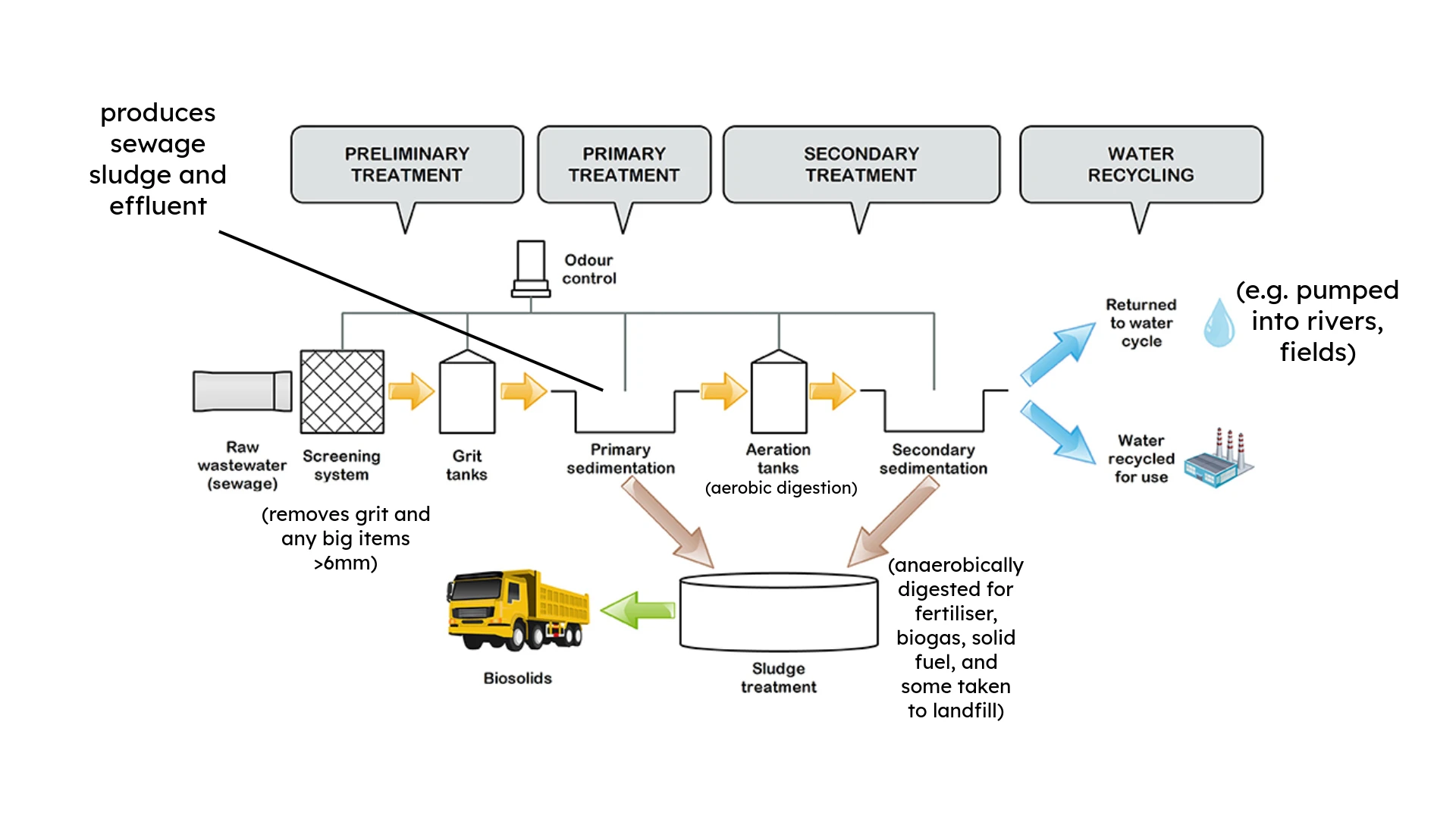 source, modified
source, modified 10.1.4 Alternative methods of extracting metals
- The Earth’s resources of metal ores are limited.
- Traditional open-cast mining takes lots of energy, makes large holes in the ground,
and produces large amounts of waste material.
- It can also cause deforestation, habitat loss, and pollution of land and water with chemicals used
in the extraction process (e.g. sulphuric acid).
- The mining can use explosives made from crude oil and the transporting, sorting, and refining of the ore
all use large amounts of energy from fossil fuels.
- Most copper is extracted by smelting copper rich ores.
- Electrolysis is used to purify impure copper obtained from smelting.
- Sulphuric acid can be used to produce copper sulphate solution from low-grade ores.
- Copper is then extracted from the solution by displacement using scrap iron.
- Phytomining and bioleaching are alternative methods of extracting metals from low-grade ores using plants and bacteria respectively.
Phytomining
- Phytomining uses plants to absorb metal compounds (often from the waste from previous mining).
- Plants are used to absorb metal compounds.
- Plants are harvested, then burned to produce ash.
- An acid is added to the ash to produce a solution containing dissolved metal ions (leachate).
- Copper can be obtained from the solution by displacement using scrap iron.
Bioleaching
- Bioleaching uses bacteria that feed on low-grade metal ores to produce leachate solutions that contain dissolved
metal compounds.
- The metal compounds can then be processed by electrolysis or displacement to obtain the metal.
