2.1 - Bonding, structure, and the properties of matter
2.1.1 Chemical bonds
- There are three types of strong chemical bonds: ionic, covalent and
metallic.
- In Ionic bonds, the positive and negative charges are attracted to each
other. It can only occur in compounds where metals are combined with non-metals.
- In Covalent bonds, the electrons are shared between the atoms. It happens
in most non-metallic elements and in most non-metal compounds.
- In Metallic bonds, the electrons are shared between the atoms, but the
atoms are held together by a network of metal ions. It occurs in metals.
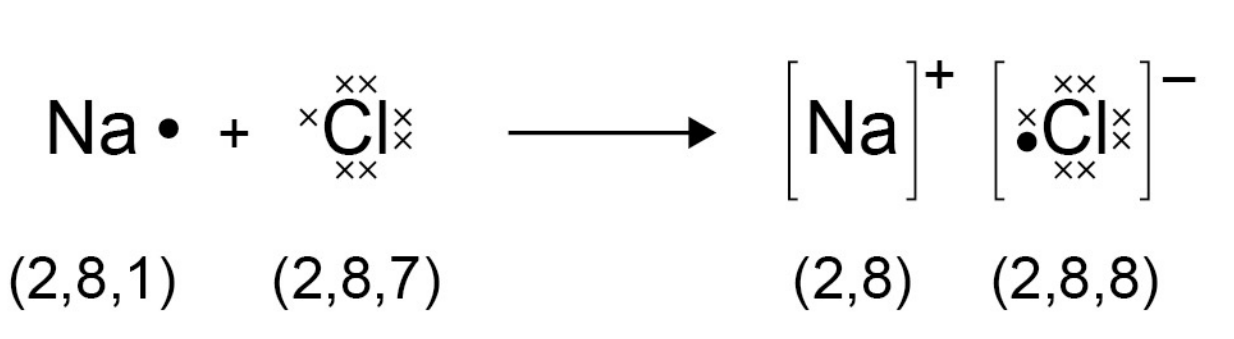
2.1.2 Ionic bonds
- Ions are atoms that have a positive or negative charge formed by the
gain or loss of electrons.
- The loss or gain of electrons gives the ion a full outer shell of electrons.
- A full outer shell needs to have 8 electrons. Metals lose electrons, and
non-metals gain electrons.
- Since most of GCSE only focuses up to Calcium, for our purposes:
- Group 1 elements lose 1 electron
- Group 2 elements lose 2 electrons
- Group 3 elements lose 3 electrons
- Group 4 elements gain 4 electrons
- Group 5 elements gain 3 electrons
- Group 6 elements gain 2 electrons
- Group 7 elements gain 1 electron
- Group 8 elements do not gain or lose electrons
- Negative ions are called anions and form when atoms gain electrons, they have more electrons than protons.
- Positive ions are called cations and form when atoms lose electrons, they have more protons than electrons.
- Here's how it's represented (image stolen from spec):
2.1.3 Ionic Structures
Giant Ionic Lattices
- This is a regular arrangement of alternating positive and negative ions.
- The ions are tightly packed together, and the electrons are evenly distributed.
- It is held together by the strong electrostatic forces of attraction between the ions, forming
the basis of ionic bonding.
- Due to the electrostatic forces, the lattice structures have high melting and boiling points.
- They allow regular shaped crystals to be formed.
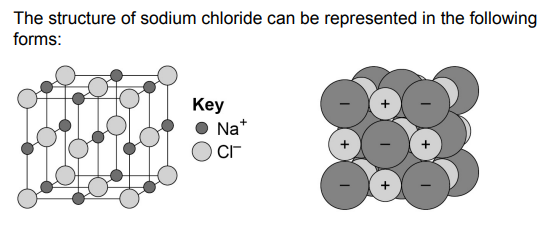 also stolen from the spec
also stolen from the spec
Ball-and-stick structure advantages/disadvantages
| Advantages | Disadvantages |
|---|---|
|
|
2.1.4 Covalent bonding
(images from spec)
- When atoms share pairs of electrons, they form strong covalent bonds.
- Covalently bonded substances may consist of small molecules, for example water (H2O).
- However, some covalently bonded substances have very large molecules, such as polymers, for example
polyethylene (C2H6).
- In addition, some covalently bonded substances have giant covalent structures, such as diamond and silicon dioxide.
Representing covalent bonds
- The covalent bonds in molecules and giant structures can be represented in the following forms:
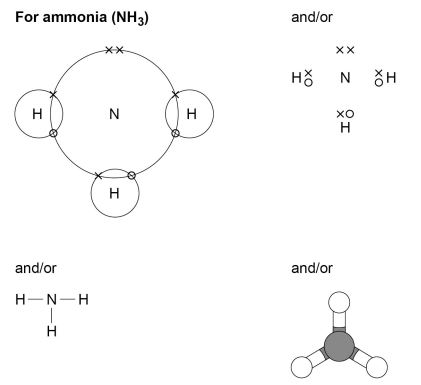
Pros and cons of each representation
| Model | Limitations |
|---|---|
| Dot and Cross Diagrams |
|
| Ball and Stick Models |
|
| 2D Diagrams |
|
| 3D Diagrams |
|
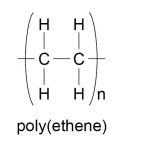
Polymers
- Polymers can be respesented in this form, where n is a large number:
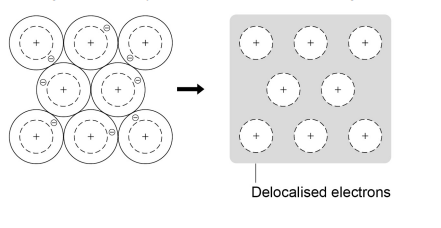
2.1.5 Metallic bonding
- Metals consist of giant structures of atoms arranged in a regular pattern.
- The electrons in the outer shell of metal atoms are delocalised and
so are free to move through the whole structure, which results strong metallic bonds
in the form of giant structures (lattices).