2.3 Structure and bonding of carbon
2.3.1 Diamond
- Diamond and carbon are allotropes of carbon.
- In diamond, each carbon atom forms four covalent bonds with other carbon atoms in a giant covalent structure, called a tetrahedron.
- Diamond is very hard due to its covalent bonds.
- It also has a very high melting point and does not conduct electricity.
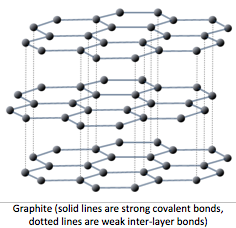
2.3.2 Graphite
- In graphite, each carbon atom forms three covelant bonds with other carbon atoms forming layers of hexagonal rings.
- These layers of hexagonal rings have no covelant bonds with other layers. This makes graphite soft as the layers can
slide over each other, despite the strong covelant bonds between the carbon atoms.
- This bonding structure also delocalises one electron from each carbon atom, making it similar to metals.
 source
source Properties of graphite
| Property | Why graphite has this property |
|---|---|
| Graphite conducts electricity | Each carbon atom is bonded to three other carbon atoms, delocalising one electron from each carbon atom. This delocalisation of electrons makes graphite a good conductor of electricity as electrons are free to pass through the material. |
| Graphite is slippery | Graphite is arranged in layers with only weak intermolecular forces holding them together, despite the strong covelant bonds between the carbon atoms. Therefore, the layers can slide over each other easily, and this makes graphite slippery. An example of this can be seen in pencils – graphite easily transferrs from pencil to paper and breaks free from the rest of the pencil. |
| Graphite has a high melting point | Graphite's strong covelant bonds between the atoms means a lot of energy is needed to break these bonds and melt the graphite. |
2.3.3 Graphene and fullerenes (and carbon nanotubes)
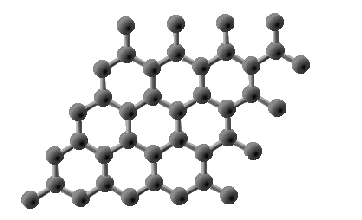
Graphene
- Graphene is simply a single layer of carbon atoms arranged in a hexagonal lattice – a single layer of graphite.
- It is the thinnest material used today, and is commonly used in electronics, in biomedical equipment, and for forming composite materials
(materials that are made from two or more combined materials).
 source
source Properties of graphene
| Property | Description | Why? |
|---|---|---|
| Structure | Single layer of carbon atoms in a hexagonal lattice, one atom thick | Each carbon is covalently bonded to three others in a 2D sheet |
| Strength | Very strong | Covalent bonds between carbon atoms are very strong and hard to break |
| Conductivity | Excellent conductor of electricity and heat | Each carbon has one delocalised electron that moves freely across the sheet |
| Thickness | Extremely thin and lightweight (only one atom thick) | It is just a single layer of atoms |
| Transparency | Transparent – lets light pass through | Only one atom thick, so it doesn’t block light |
| Flexibility | Flexible and can be bent or stretched without breaking | The sheet can bend while strong covalent bonds stop it from snapping |
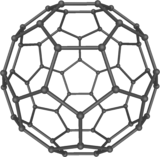
Fullerenes
- Fullerenes are molecules of carbon atoms with hollow shapes/tubes.
- Their structure is based on hexagonal rings of carbon atoms, however they may also contain rings
with five or seven carbon atoms.
- The first fullerene to be discovered was Buckminsterfullerene (or "bucky ball") (C60) which has a spherical shape:
 source
source Properties of fullerenes
| Property | Description | Why? |
|---|---|---|
| Structure | Molecules of carbon shaped like hollow spheres or tubes (e.g. C60) | Carbon atoms form hexagons and pentagons joined into a closed cage |
| Strength | Quite strong but not as strong as graphene/nanotubes | Covalent bonds hold atoms together, but curved structure is weaker than flat layers |
| Conductivity | Poor conductor | Delocalised electrons are trapped within the molecule and cannot move freely between molecules |
| Size/Surface area | Very small, high surface area | Hollow spherical/tubular shape gives large surface area relative to volume |
| Uses | Drug delivery, catalysts, lubricants | Can carry molecules inside; high surface area for reactions; slippery molecules reduce friction |
Carbon nanotubes
- Carbon nanotubes are cylindrical fullerenes.
- They have very high length to diameter ratios, which makes them useful for nanotechnology, electronics and materials.
| Property | Description | Why? |
|---|---|---|
| Structure | Very long, thin cylinders of carbon atoms (rolled-up graphene sheets) | Hexagonal carbon lattice forms a tube shape |
| Strength | Extremely strong and stiff | Covalent bonds between carbon atoms are very strong and continuous along the tube |
| Conductivity | Excellent conductor of electricity and heat | Delocalised electrons can move freely along the tube |
| Size/Surface area | Very small diameter, high length-to-diameter ratio | Nanoscale tubes give very high surface area and unique mechanical properties |
| Uses | Reinforcing materials, electronics, nanotechnology | Strength makes composites tougher; conductivity useful in circuits; small size for nanoscale applications |
