5.2 Chemical cells and fuel cells
5.2.1 Cells and batteries
- Cells are the basic units of chemical energy storage.
- Chemicals inside them react to produce electrical energy.
- The voltage produced by a cell is dependent upon a number of
factors including the type of electrode and electrolyte.
- A simple cell can be made by connecting two different metals in
contact with an electrolyte.
- Batteries consist of two or more cells connected in series,
which produces a greater voltage.
- In non-rechargable cells (primary cells), the chemical reactions stop when one of
the reactants is depleted. An example of this is a standard household
alkaline battery (like your basic Duracell or Energizer non-rechargable).
- In rechargable cells (secondary cells), the chemical reactions also stop when one of the
reactants is depleted, but the cell can be recharged by passing an electric
current in the opposite direction to the output (cells produce DC).
Cell Diagram
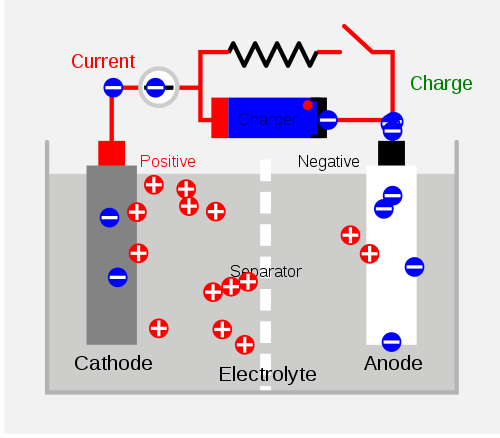 source
source
If, in this diagram, the cathode was Zinc and the anode was Copper, then, in discharging, the Zinc
would be oxidised in the half-equation Zn -> Zn2 + 2e-
and the Copper would be reduced in the half-equation Cu2 + 2e- -> Cu.
Shown in the diagram is charging, therefore this is reversed.
5.2.2 Fuel Cells
- Fuel cells are supplied by an external source of fuel (e.g. hydrogen)
and oxygen or air.
- The fuel is oxidised electrochemically within the fuel cell to
produce a potential difference (a voltage).
- The overall reaction in a hydrogen fuel cell involves the oxidation of
hydrogen to produce water.
Fuel Cell Diagram
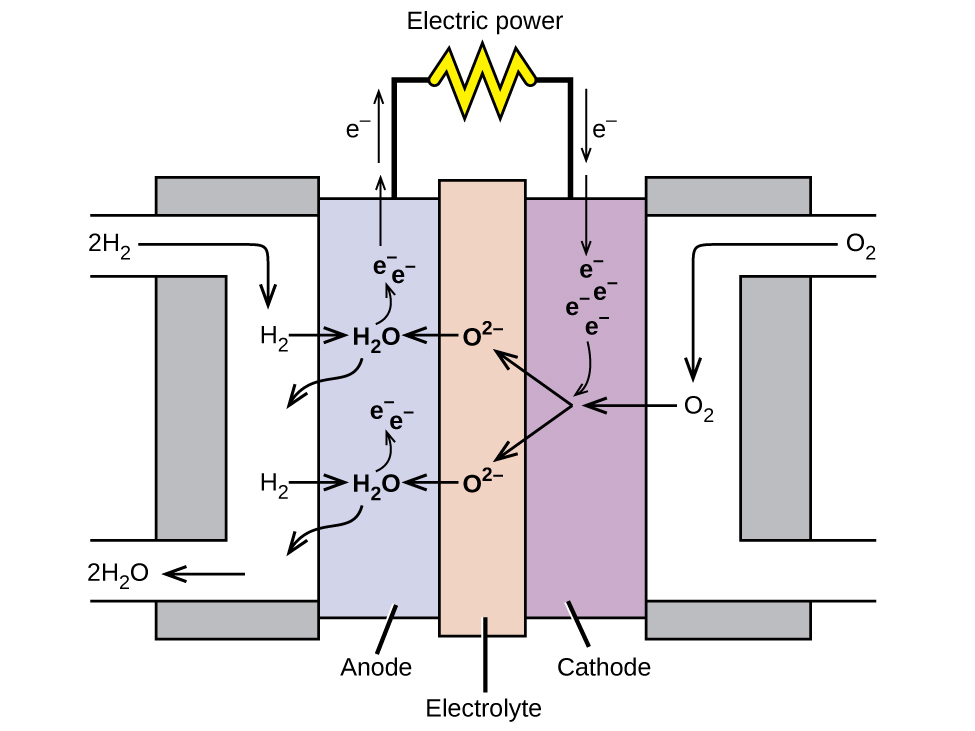 source
source Electrode Reaction Half Equations
- Hydrogen enters at the anode where it is oxidised and oxygen enters at the
cathode where it is reduced.
- There are two half-equations for the fuel cell:
4e- + O2 + 2H2O -> 4OH- (reduction [cathode])
2OH- + H2 -> 2H2O + 2e- (oxidation [anode])
- And this makes the full equation:
2H2 + O2 -> 2H2O
Advantages and Disadvantages of Fuel Cells
| Advantages | Disadvantages |
|---|---|
| They do not produce pollution (only water vapour). | Hydrogen is flammable. |
| They produce lots of energy per kilogram (more than petrol or diesel). | Fuel cells are affected by low temperature (however batteries are too). |
| No batteries to dispose of or produce. | Materials to produce fuel cells are expensive. |
| Energy is produced as long as fuel is available (chain reaction). | High pressure tanks are needed to store and transport hydrogen, which also requires cooling to very low temperatures in order to compress the hydrogen and this is expensive. |
| No moving parts. | Using hydrogen very inefficient (often only half as efficient as batteries or worse due to energy required for compression, storage, transport and fuel cells just being less efficient). |
