Chemistry 6.1 Rate of reaction
6.1.1 Calculating rates of reactions
The rate of a chemical reaction can be found by measuring the quantity of a reactant used or the quantity of product formed over time:
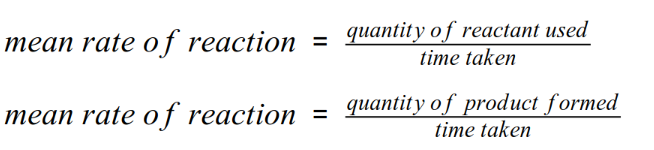
- The quantity of reactant or product can be measured by the mass in
grams, moles, or by a volume in cm3.
- The units of rate of reaction maybe given as g/s, mol/s, or cm3/s
Example Calculations
Example 1: Calculate the rate of reaction for the decomposition of hydrogen peroxide (H₂O₂) into water (H₂O) and oxygen (O₂).
Chemical equation (balanced): 2 H₂O₂ → 2 H₂O + O₂
- What is being measured? The quantity of reactant used: 0.5 mol of H₂O₂ decomposes.
- Time interval: 10 s.
- Formula: rate = (quantity of reactant used or product formed) / (time taken).
- Substitute the values:
rate = 0.5 mol / 10 s - Do the division:
rate = 0.05 mol/s - Units and interpretation: The rate is 0.05 mol s⁻¹. This means on average 0.05 moles of H₂O₂ are consumed each second over the measured period.
Example 2: Calculate the rate of reaction for the formation of ammonia (NH₃) from nitrogen (N₂) and hydrogen (H₂). Give the rate in g/s and also show the equivalent in mol/s.
Chemical equation (balanced): N₂ + 3 H₂ → 2 NH₃
- What is being measured? The quantity of product formed: 34.0 g of NH₃ (measured mass).
- Time interval: 20 s.
- Rate in g/s (direct):
rate = mass of product / time = 34.0 g / 20 s = 1.7 g/s. - Units and interpretation: The reaction produces 1.7 grams of NH₃ each second on average during the measured period.
- Optional — convert to mol/s:
Molar mass of NH₃ = 14.01 (N) + 3×1.008 (H) ≈ 17.03 g/mol.
Moles of NH₃ formed = 34.0 g ÷ 17.03 g/mol ≈ 1.997 mol ≈ 2.00 mol (to three significant figures).
rate in mol/s = 2.00 mol / 20 s = 0.100 mol/s.
6.1.2 Factors which affect the rates of chemical reactions
| Factor | Increases frequency of collision | Increases energy of particles |
|---|---|---|
| Temperature | Yes | Yes |
| Surface area to volume ratio | Yes | No |
| Increase concentration (solutions) | Yes | No |
| Increase pressure (gases) | Yes | No |
| Add a catalyst | No | No |
6.1.3 Collision theory and activation energy
- Collision theory states that chemical reactions can occur only when reacting
particles collide with each other and with sufficient energy.
- The minimum amount of energy that particles must have to react is called
the activation energy.
6.1.4 Catalysts
- Catalysts, while increasing the rate of a reaction, but are not used
up during the reaction.
- This means that the catalyst is still there, unchanged, after the reaction.
- Different reactions need different catalysts.
- An example of a catalyst is enzymes in biological systems.
- Catalysts increase the rate of reaction by providing a different
pathway for the reaction that has a lower activation energy.
Energy profile diagram
(stolen from the spec)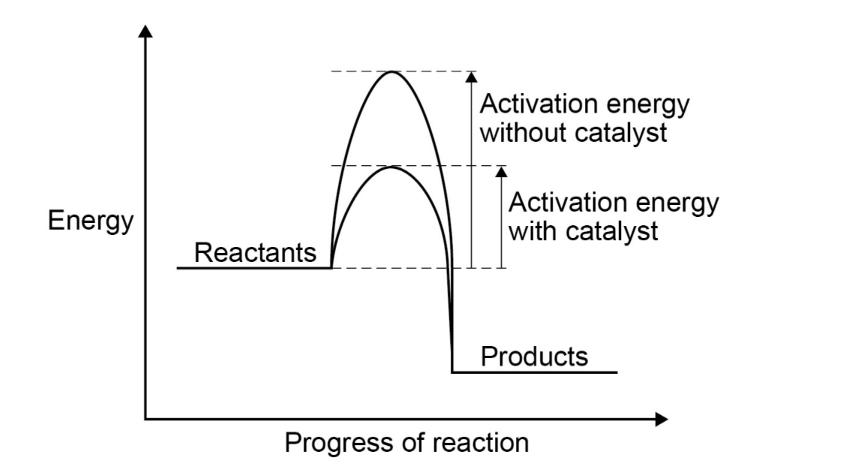
- According to collision theory, a catalyst lowers the activation energy so
a greater proportion of collisions result in a successful reaction.
- The frequency of collisions remains the same.
Catalyst Examples
| Catalyst | Use | Why is this an important use of catalysts? |
|---|---|---|
| Enzymes (as biological catalysts). | Washing detergent | Enzymes are used to break down food that may be on clothes or in the environment. This makes it a lot easier for the washing machine to remove it from the clothes. |
| Platinum, Palladium, and Rhodium | Catalytic converters | Platinum and Palladium: Help oxidise carbon monoxide (CO) and
unburned hydrocarbons into carbon dioxide (CO₂) and water (H₂O). Rhodium: Focuses on reducing nitrogen oxides (NOₓ) into nitrogen (N₂) and oxygen (O₂). |
| A finely divided iron catalyst, often enhanced with promoters like potassium oxide (K₂O) and alumina (Al₂O₃). | Haber process | The catalyst reduces the energy, and therefore heat, required for the reaction to occur, making it a lot cheaper to produce. The Haber-Bosch process makes ammonia, one of the world's most important chemicals, as it is used as fertiliser. |
| Platinum, palladium, rhodium, or nickel. | Hydrogenation - making spreads from vegetable oils | Hydrogenation can turn liquid vegetable oils (unsaturated fats) into solid or semi-solid fats, often used in food production. |
| Calcium Carbonate | Biodiesel | Calcium Carbonate is used to make biodiesel, which is a renewable fuel made from vegetable oils. This is important as use of biodiesel can reduce carbon emissions and the amount of new oil that needs to be drilled for. |
| Zeolites | Petroleum refining | In the chemical and petrochemical industries, zeolites are used as catalysts in processes like oil refining and the production of fuels, making production cheaper and more efficient. |
