6.2 Reversible reactions and dynamic equilibrium
6.2.1 Reversible reactions
In some chemical reactions, the products of the reaction can react
to produce the original reactants.
Such reactions are called reversible reactions.
They can be represented by:
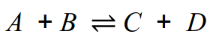
Equilibrium: When the rate of the forwards reaction is equal to the rate of
the reverse reaction.
This can only happen in a closed system, meaning that
no matter can enter or leave the system.
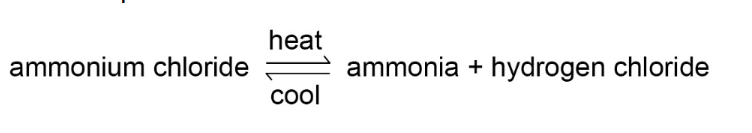
- The direction of reversible reactions can be changed by changing
the conditions. For example:
 - Other examples include litmus, phenolphlatein, and pretty much any other indicator.
- Other examples include litmus, phenolphlatein, and pretty much any other indicator.
6.2.2 Energy changes and reversible reactions
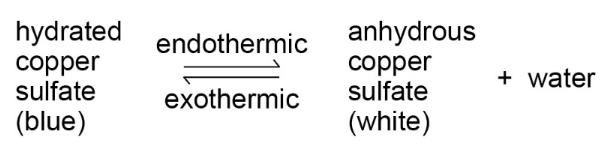
- If a reversible reaction is exothermic in one direction, it is endothermic in the opposite direction.
- As energy cannot be created or destroyed, the same amount of energy is transferred in each case.
- For example:
- On an energy profile diagram, this results in a "mirror image", like this one:
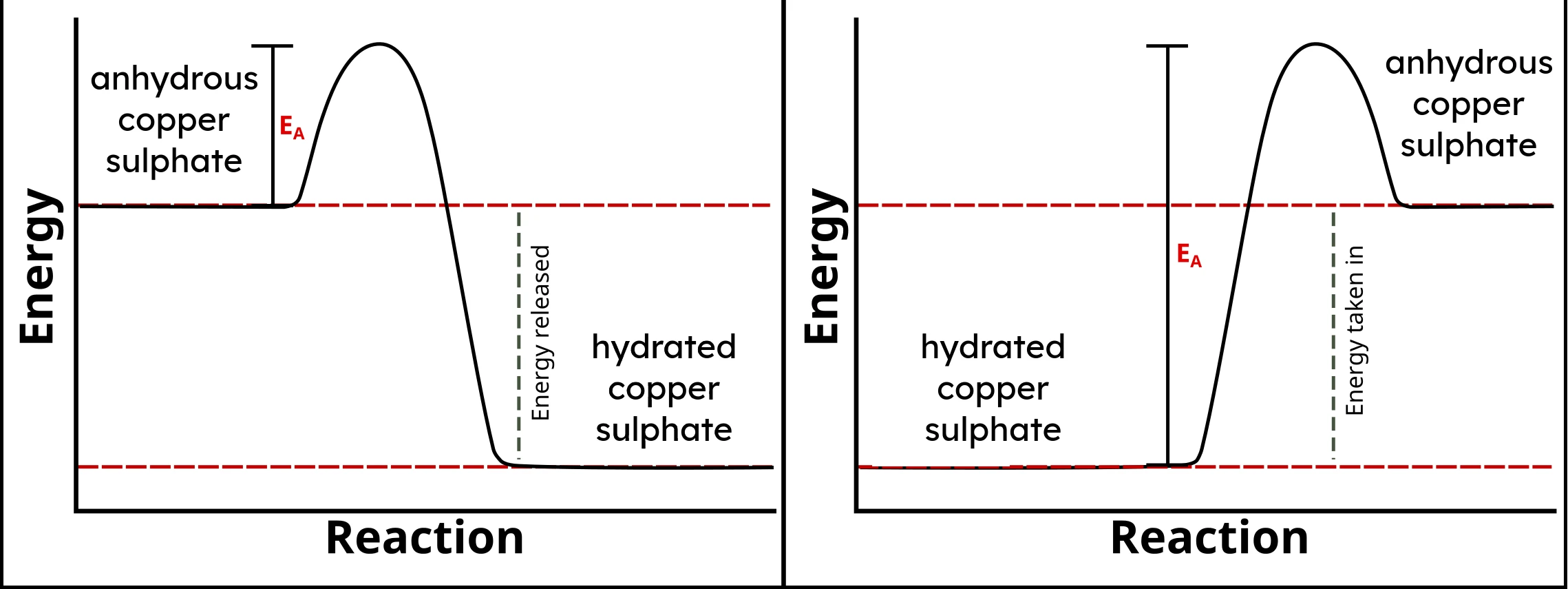 source, modified
source, modified
6.2.3 Equilibrium
(stolen from spec)
When a reversible reaction occurs in apparatus which prevents the
escape of reactants and products, equilibrium is reached when the
forward and reverse reactions occur at exactly the same rate.
6.2.4 The effect of changing conditions on equilibrium
The relative amounts of all the reactants and products at equilibrium
depend on the conditions of the reaction.
The effects of changing conditions on a system at equilibrium can
be predicted using Le Chatelier's Principle.
Le Chatelier's Principle
Le Chatelier's Principle: if a change in conditions is applied
to a system in dynamic equilibrium, the system will respond to counteract
that change.
Therefore, we can predict what might happens when we change the conditions
of a system.
This is a bit like Newton's Third Law, but in chemistry.
6.2.5/6/7 The effect of changing concentration, temperature, and equilibrium
Concentration
- If the concentration of one of the reactants or products is changed, the system is no longer at equilibrium.
- The concentrations of all the substances will change until equilibrium is reached again.
- If the concentration of a reactant is increased, more products will be formed until equilibrium is reached again.
- If the concentration of a product is decreased, more reactants will react until equilibrium is reached again.
Temperature
- Temperature is a measure of average (mean) thermal energy of the particles in a system.
- Increasing temperature increases the thermal energy of the system.
- The equilibrium will shift to reduce the temperature.
- This works the other way around too.
- If the temperature of a system at equilibrium is increased, the relative amount of products
at equilibrium increases for an endothermic reaction and decreases for an exothermic reaction.
- The reverse is true for a lower temperature.
Pressure
- If a reversible reaction involves changing the number of gas molecules,
altering the pressure can affect the equilibrium mixture.
- An increase in pressure favours the reaction that forms fewer molecules, while a
decrease in pressure favours the reaction that forms more molecules.
