7.1 Carbon compounds as fuels and feedstock
7.1.1 Crude oil, hydrocarbons and alkanes & 7.1.2 Fractional distillation and petrochemicals & 7.1.3 Properties of hydrocarbons & 7.1.4 Cracking and alkenes
- Properties of hydrocarbons depend on the size of their molecules.
- Boiling Point: Temperature at which the liquid boils or the gas condenses. Bigger size = higher boiling point.
- Volatility: The tendency to turn into a gas. Bigger size = lower volatility.
- Viscosity: How easily it flows. Bigger size = higher viscosity (thicker).
- Flammability: How easily it burns. Bigger size = lower flammability.
| Molecular formula | Boiling point |
|---|---|
| CH4 | -161°C |
| C2H6 | -89°C |
| C3H8 | -44°C |
| C4H10 | -0.5°C |
| C5H12 | 36°C |
| (and so on...) | (and so on...) |
- All hydrocarbons have strong covalent bonds between the carbon atoms,
but very weak intermolecular forces between the molecules.
- The more atoms the molecule is made out of, the higher the
strength of the intermolecular forces.
Crude oil
- Crude oil is a finite resource found in rocks. Crude oil is the remains
of an ancient biomass consisting mainly of plankton that was buried
in mud.
- Crude oil is a mixture of a very large number of compounds. Most of
the compounds in crude oil are hydrocarbons, which are molecules
made up of hydrogen and carbon atoms only.
- Crude oil is mostly made up of alkanes.
Fractional Distillation
- Crude oil is separated into hydrocarbons with similar boiling points called fractions.
- Fractions are mixtures of hydrocarbons with similar physical properties.
- This process is called fractional distillation.
- Crude oil is fed into the oven (bottom left of the image) and vapourised to produce a gas,
which is then fed into the fractionaiting column.
- The gas enters the tower that is hot at the bottom and cool at the top.
- Molecules cool and condense at different heights, as molecules have different boiling points.
- Smaller molecules are collected higher up the tower.
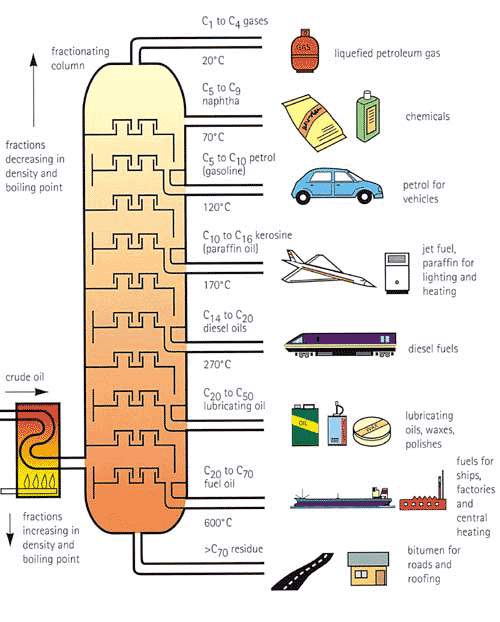 source
source Complete vs Incomplete combustion
- Complete Combustion is where all the hydrocarbons are burnt in excess oxygen.
- Methane + oxygen -> carbon dioxide + water
- Incomplete Combustion is where the hydrocarbons are burnt in limited oxygen.
- This produces CO (carbon monoxide) instead of CO2.
- Carbon monoxide is a poisonous gas. It binds to haemoglobin irreversibly in red blood cells and
prevents oxygen from binding.
- Carbon particulates can also be produced, some of which will be nanoparticles and some will be
coarse particles. This is bad for long-term health and can increase the risk of lung diseases.
Propane Equations
Complete combusion: C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(g)
Incomplete combustion (CO): C3H8(g) + 3.5O2(g) → 3CO(g) + 4H2O(g)
Incomplete combustion (C): C3H8(g) + 2O2(g) → 3C3 + 4H2O(g)
Cracking
- Hydrocarbons can be brown down (cracked) to produce smaller more useful molecules.
- C10H22 → C5H12 + C3H8 + C2H4
- The products made are more useful than the starting hydrocarbons.
Types of Cracking
| Catalytic cracking | Steam cracking |
|---|---|
|
|
