7.2 Reactions of Alkenes and Alcohols
7.2.1 Structure and formulae of alkenes
(stolen from spec)
- Alkenes are hydrocarbons with a double carbon-carbon bond.
- The general formula for the homologous series of alkenes is CnH2n.
- Successive members of a homologous series differ by an extra carbon and two extra hydrogen atoms.
- Alkene molecules are "unsaturated" because they contain two fewer
hydrogen atoms than the alkane with the same number of carbon atoms.
7.2.2 Reactions of alkenes
Addition reactions
Addition reactions are where we take two reactants and combine them to make one product.
Alkenes react with hydrogen, water, and halogens by the addition of atoms across the carbon-carbon
double bond.
- Hydrogenation: Adding hydrogen.
- Chlorination: Adding chlorine.
- Bromination: Adding bromine.
- Hydration: Adding water.
Hydrogenation
nickel catalyst, 150°C
ethene + hydrogen → ethane
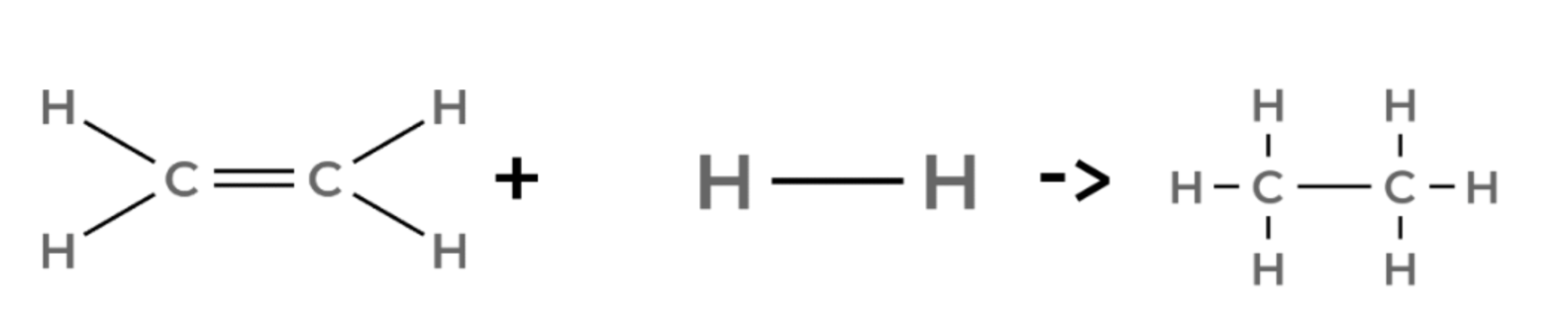 propene + hydrogen → propane
propene + hydrogen → propane
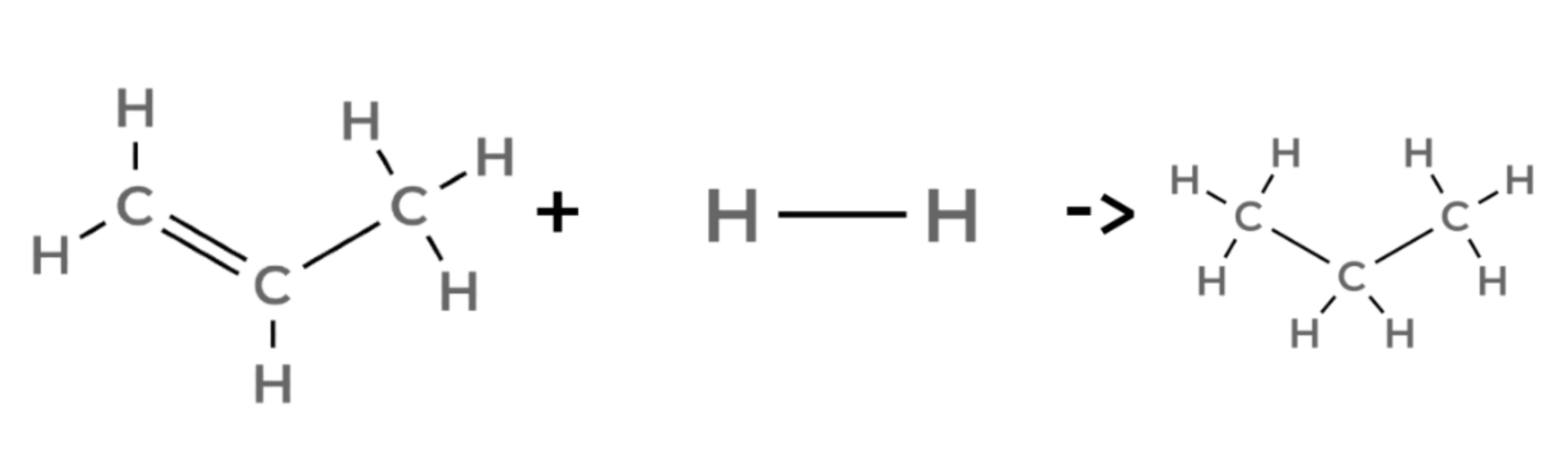
Halogenation
ethene + chlorine → 1,2-dichloroethane
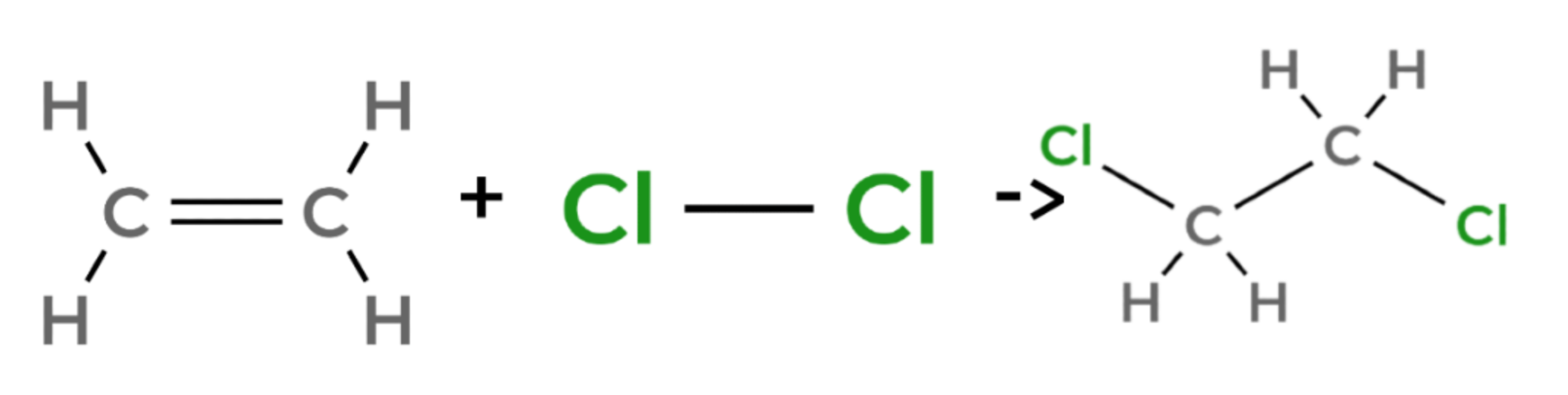 propene + bromine → 1,2-dibromopropane
propene + bromine → 1,2-dibromopropane
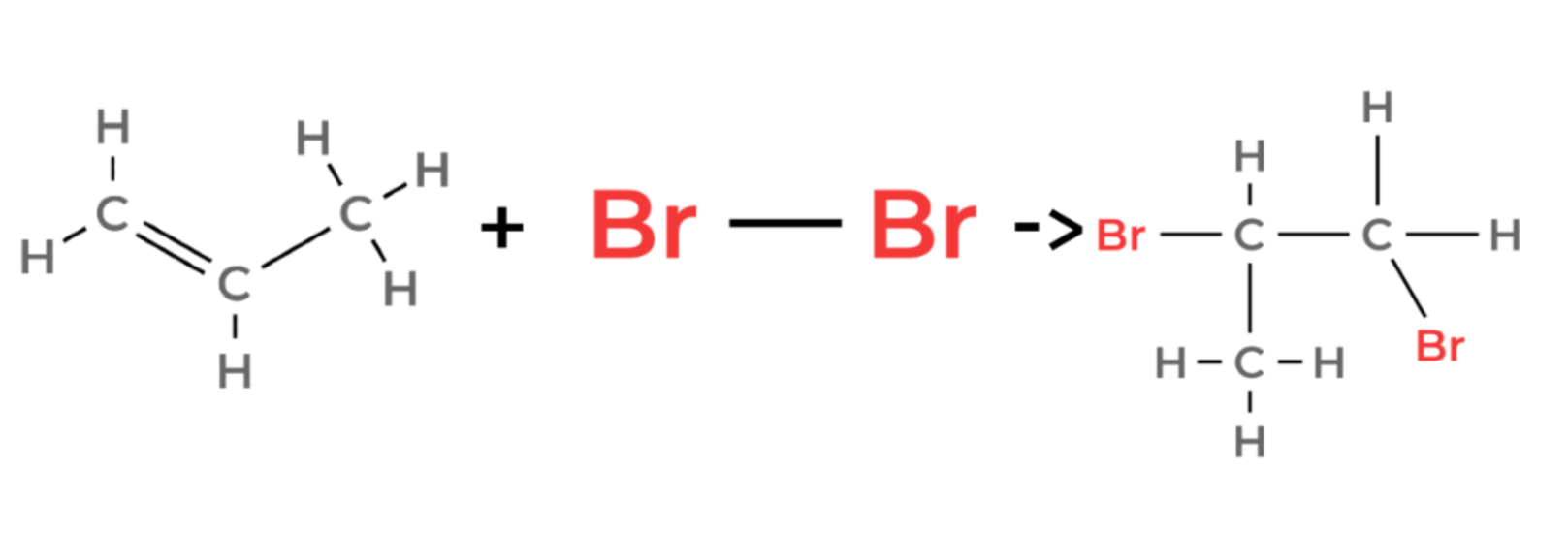
Hydration
ethene + water → ethanol
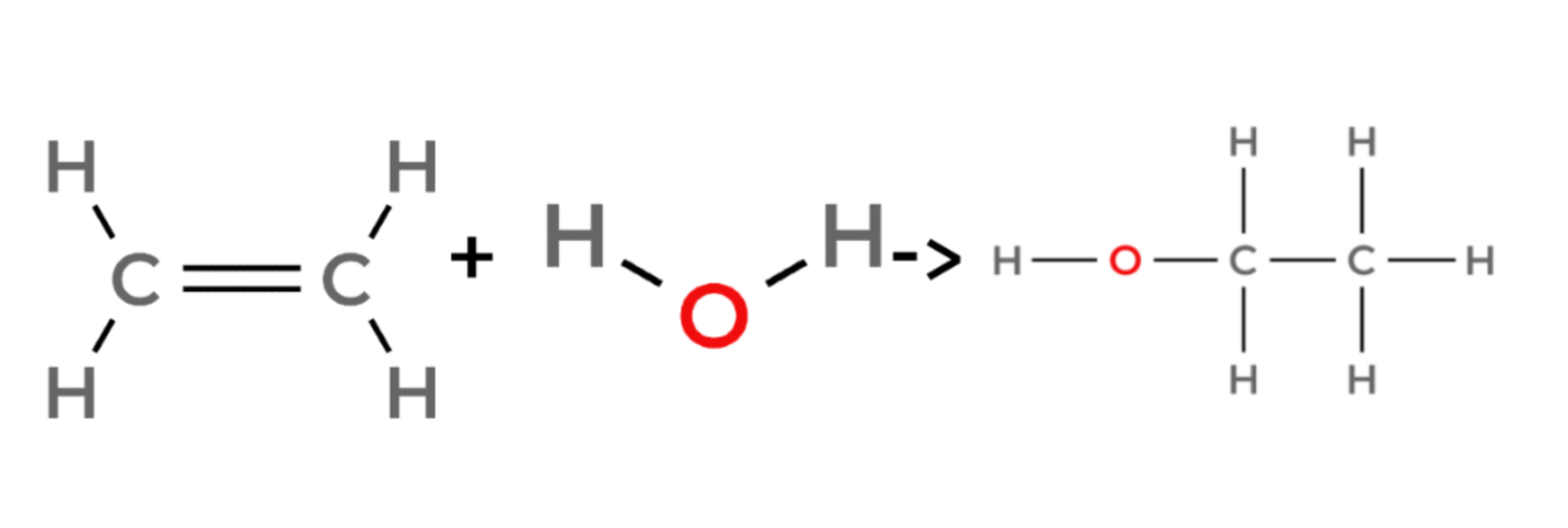 propene + water → propanol
propene + water → propanol
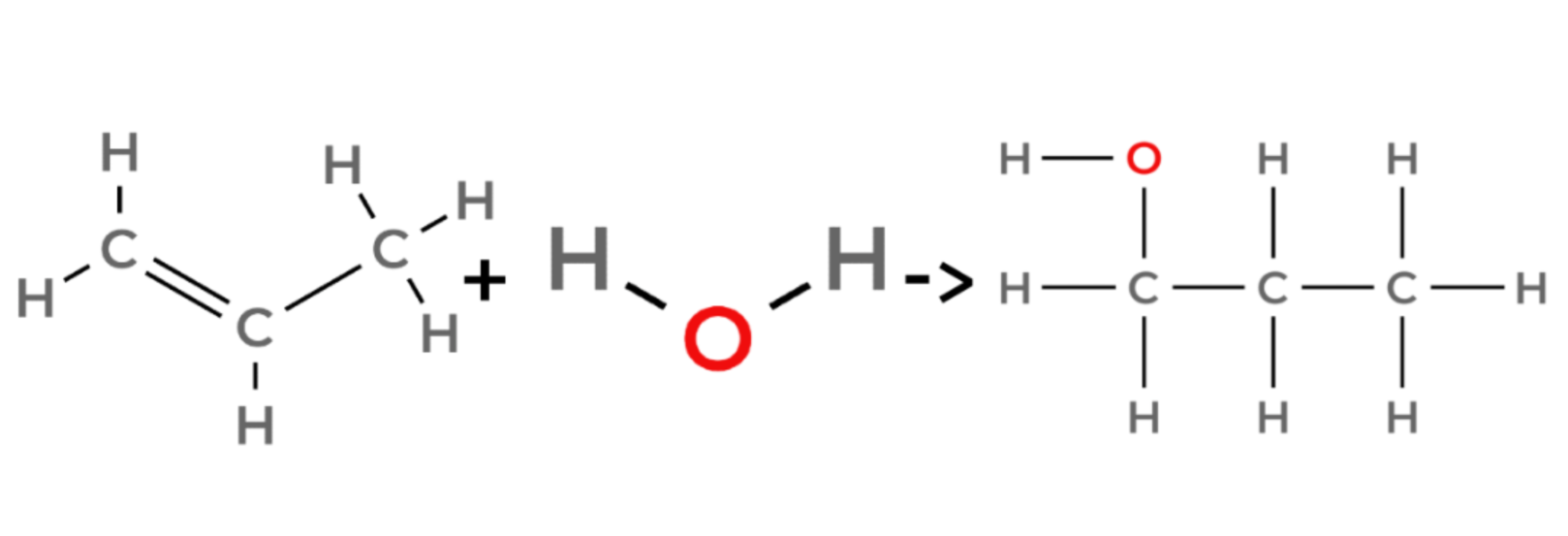
7.2.3 Alcohols
- Keywords: oxidation, anaerobic, fermentation.
- Oxidation: Adding oxygen atoms; the loss of hydrogen atoms or the loss of electrons.
- Anaerobic: In the absence of oxygen.
- Fermentation: A chemical process where microorganisms like yeast break
down sugars such as glucose into ethanol and carbon dioxide.
- Alcohols contain the functional group -OH.
Methanol, ethanol, propanol and butanol are the first four members
of a homologous series of alcohols.
These are those alcohols.
Uses
- Alcohols are in many everyday products:
- Alcoholic drinks
- Solvents
- Perfumes, aftershaves, mouthwash
- Fuels
Complete combustion of alcohols
- 2CH3OH (methanol) + 3O2 → 2CO2 + 4H2O
- C2H5OH (ethanol) + 3O2 → 2CO2 + 3H2O
- 2C3H7OH (propanol) + 9O2 → 6CO2 + 8H2O
Incomplete combustion of alcohols
- 2CH3OH (methanol) + O2 → 4H2O + 2C
- C2H5OH (ethanol) + 2O2 → 3H2O + 2CO
- C3H7OH (propanol) + 2O2 → 4H2O + CO + 2C (or other ways,
you can balance this quite a few days)
Oxidation reactions
- Ethanol + oxygen → ethanoic acid (vinegar) + water
- C2H5OH + 2[O] → CH3COOH + H2O
- (oxygen is the oxidising agent)
- Pottassium dichromate (K2Cr2O7) is an oxidising agent
which can be used to oxidise alcohols.
7.2.4 Carboxylic acids
- Carboxylic acids have the functional group -COOH.
- The first four members of a homologous series of carboxylic acids
are methanoic acid, ethanoic acid, propanoic acid and butanoic acid.
- As a reminder, a strong acid is fully ionised in water (e.g. HCl).
- HCl → H+ + Cl-
- A weak acid is only partially ionised in water (e.g. ethanoic acid).
- CH3COOH ⇌ H+ + CH3COO-
- Carboxylic acids are weak acids. They give off relatively few H+ ions in solution and
have a higher pH.
- Here is the functional group in the context of ethanoic acid:
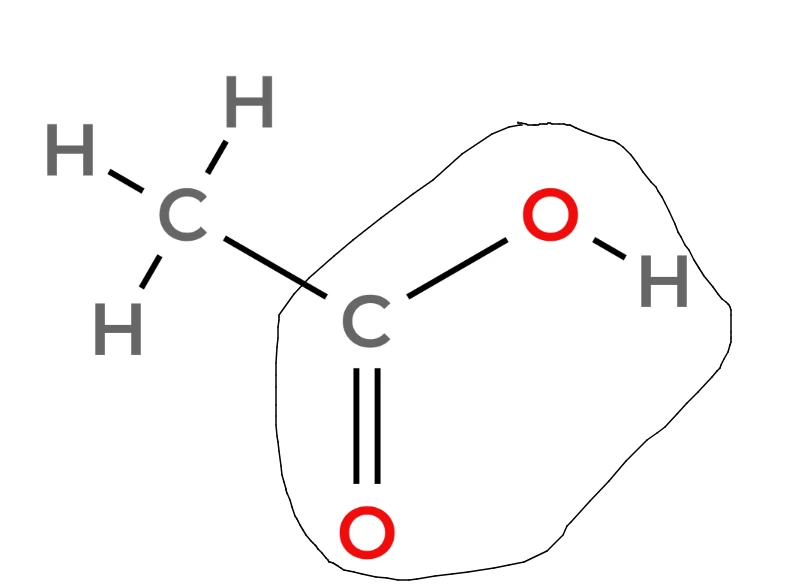
Reactions of carboxylic acids with carbonates
- When we react carboxylici acids with a metal carbonate, it reacts as a typical acid producing salt,
carbon dioxide, and water.
- ethanoic acid + sodium carbonate → sodium ethanoate + carbon dioxide + water
- 2CH3COOH(aq) + Na2CO3(s) → 2CH3COONa(aq) + CO2(g) + H2O(l)
- Reactions with carboxylic acids have a lower rate of reaction because:
- There is a lower concentration of H+ ions
- Collisions are less frequent
Making esters
- Carboxylic acids will react with alcohols to produce esters and water.
- A catalyst is needed (in this case sulphuric acid).
- Esters are volatile compounds with distinctive smells, often fruity.
- They are used in perfumes and food flavourings.
- The one you need to know is ethyl ethanoate, made from ethanol and ethanoic acid.
- The yield is low because the reaction is reversible.
- ethanoic acid + ethanol ⇌ ethyl ethanoate + water
- CH3COOH + C25H5OH ⇌ CH3COOC2H5 + H2O
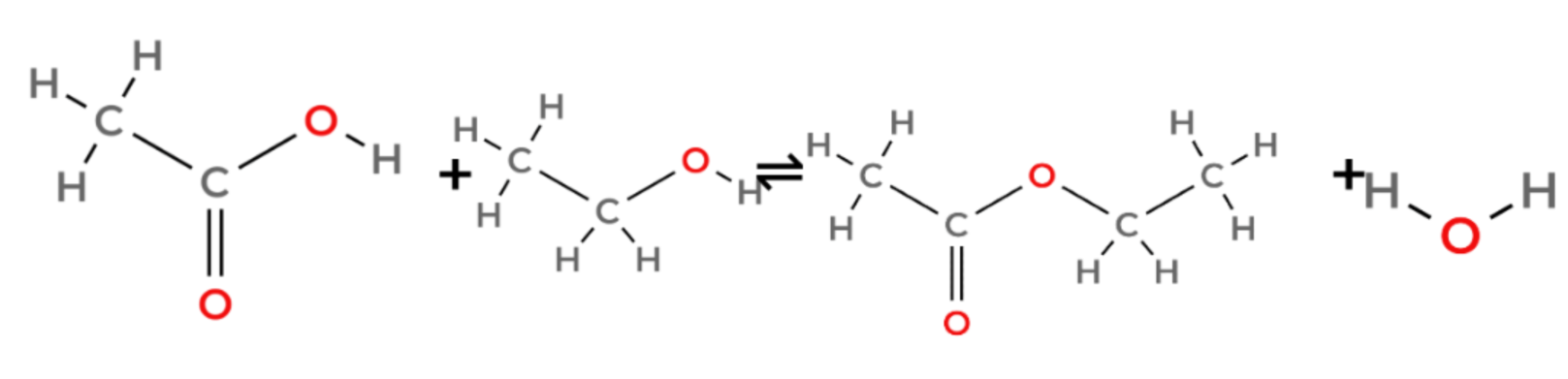 (sorry for how bad this image is, it was really difficult to make :c)
(sorry for how bad this image is, it was really difficult to make :c)
Uses of esters and carboxylic acids
- Esters are used in:
- Perfumes
- Food flavourings
- Solvents for paints and glue
- Food preservatives (e.g. vinegar)
- Manufacture of soaps and detergents
- Manufacture of pharmaceuticals (e.g. aspirin)
Esterification
methanol + methanoic acid ⇌ methyl methanoate + water
CH3OH + HCOOH ⇌ HCOOCH3 + H2O
- This is a condensation reaction.
propanoic acid + ethanol ⇌ ethyl propanoate + water
C2H5COOH + C2H5OH ⇌ C2H5COOC2H5 + H2O
(and many others, but you get the idea)
