7.3 Synthetic and naturally occurring polymers
7.3.1 Addition polymerisation
- One of the most important ways that chemicals from crude oil
are used is to make polymers.
- Polymers are substances made from very large molecules made up
of many smaller repeating units (monomers).
- Polymers can be natural (e.g. starch, cellulose, proteins) or
synthetic (e.g. poly(ethene), poly(propene)).
- Most synthetic polymers are made from monomers that contain
carbon-carbon double bonds (alkenes).
- Alkenes can be used to make polymers such as poly(ethene) and
poly(propene) by addition polymerisation.
- In addition polymerisation reactions, many small molecules
(monomers) join together to form very large molecules (polymers), and nothing else.
- In addition polymers the repeating unit has the same atoms as the
monomer because no other molecule is formed in the reaction.
Equations
Ethene → Poly(ethene)
n(CH2=CH2) → (–CH2–CH2–)n 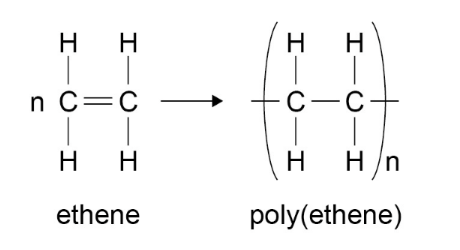 Propene → Poly(propene)
Propene → Poly(propene)
n(CH2=CHCH3) → (–CH2–CH(CH3)–)n 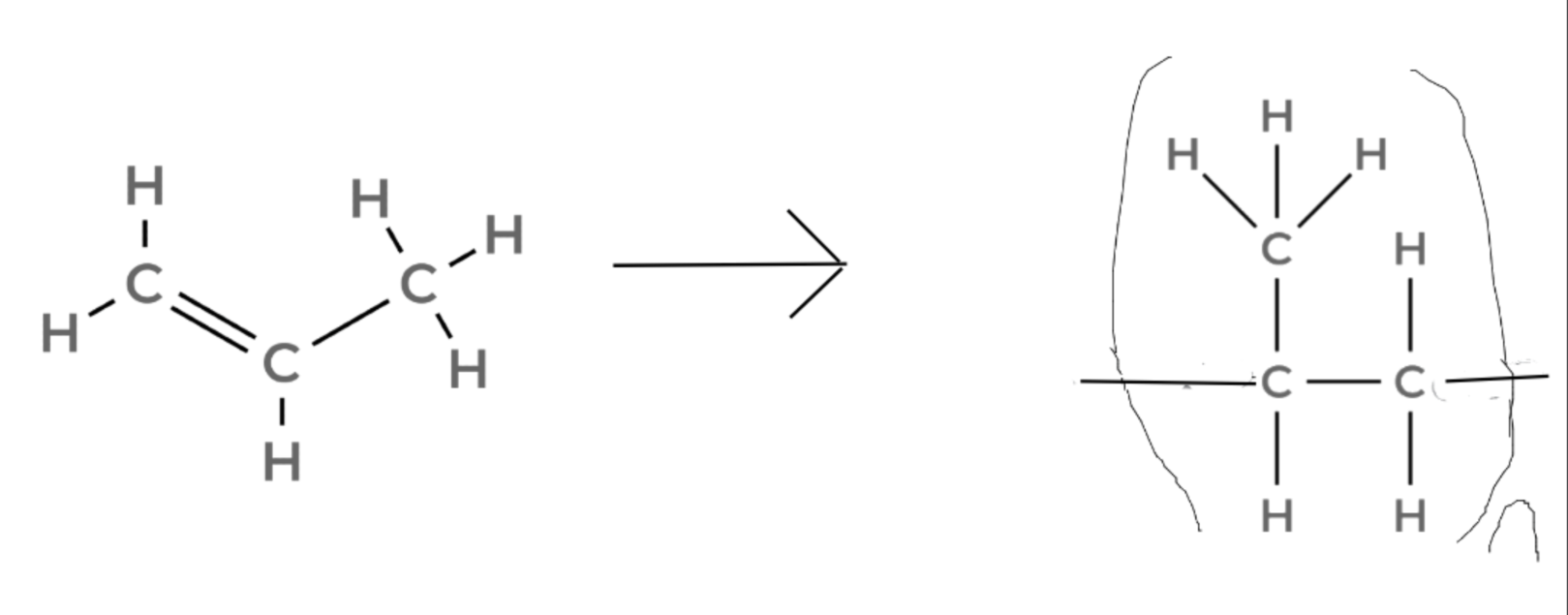
How we use polymers
- Ethene monomers can be used to make poly(ethene), which is used to make
plastic bags and bottles.
- It is cheap, flexible, and strong.
- Propene monomers can be used to make poly(propene), which is used to make
ropes, carpets, and crates.
- It is strong, durable, and has a high melting point.
7.3.2 Condensation polymerisation
- Condensation polymerisation involves monomers with two functional groups.
- This also results in formation of two products: the polymer and a small molecule
(often water).
- Therefore, condensation polymerisation is many monomers with two functional
groups joined together to make a very long repeating molecule and another small molecule
such as H2O or HCl.
An example of a condensation reaction
methanol + butanoic acid → methyl butanoate + water
CH3OH + CH3CH2CH2COOH → CH3CH2CH2COOCH3 + H2O
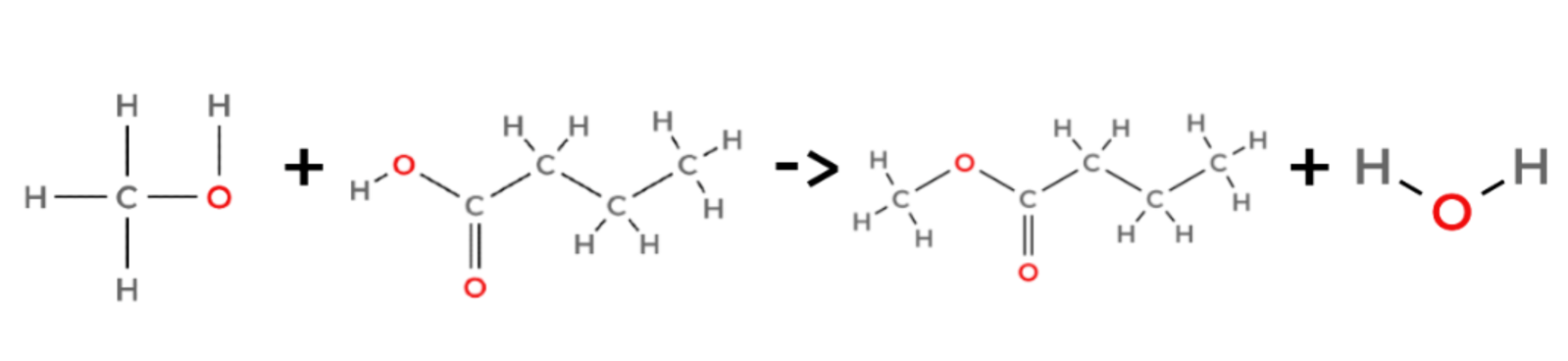 An H2SO4 catalyst is required.
An H2SO4 catalyst is required.
Polyesters
- Polyesters are formed from dicarboxylic acids and diols (alcohols with two -OH groups).
- The -COOH group of the dicarboxylic acid reacts with the -OH group of the diol to form an ester link (-COO-) and a molecule of water.
- The repeating unit in the polyester contains atoms from both monomers, unlike in
addition polymerisation where the repeating unit contains only atoms from the monomer.
- An example of a polyester is poly(ethene terephthalate) (PET), which is made from
the dicarboxylic acid benzene-1,4-dicarboxylic acid and the diol ethane-1,2-diol.
- Polyesters are used to make fibres for clothing and plastic bottles.
- Here is how to make an example polyester:
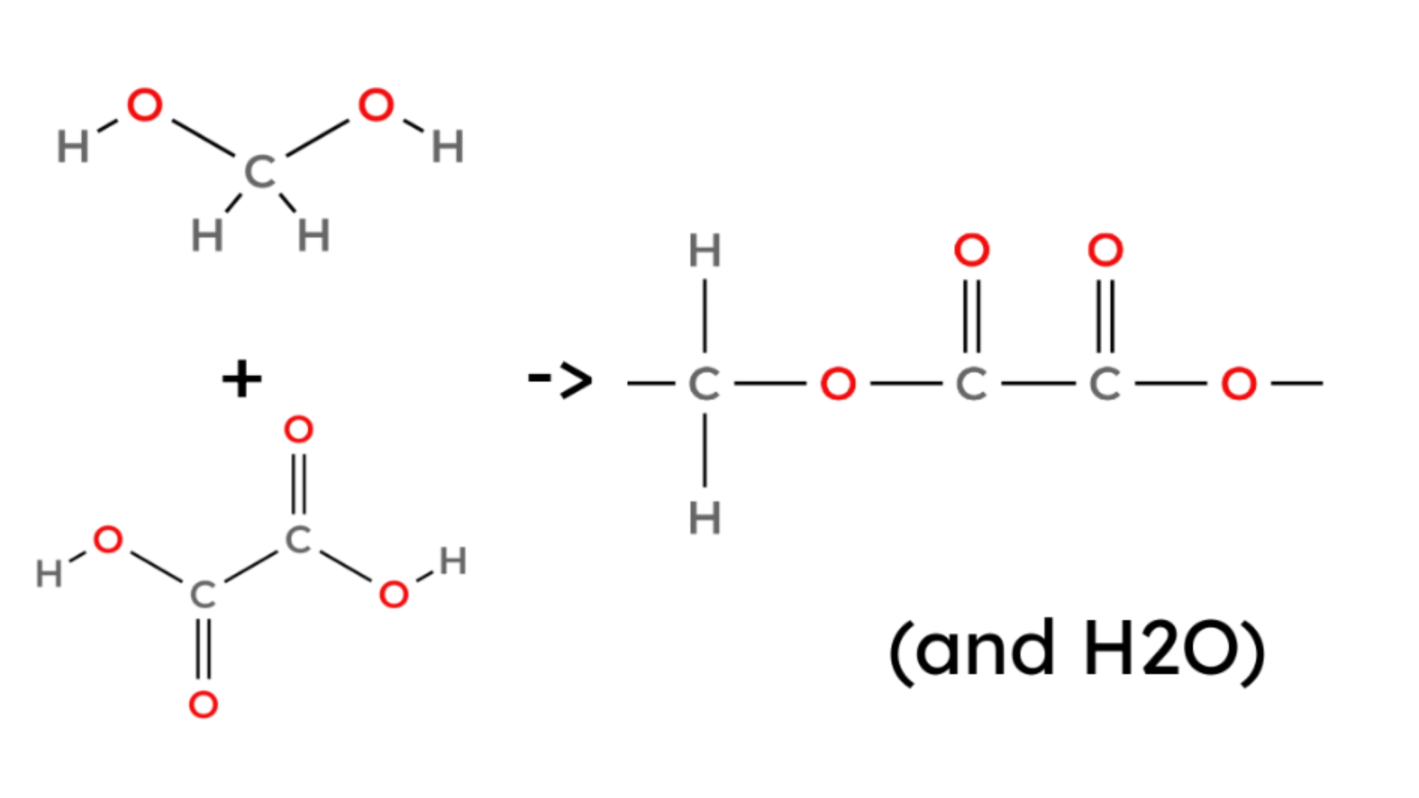 (methanediol + ethanedioic acid → poly(methanediol ethanedioate) + water)
- And another:
(methanediol + ethanedioic acid → poly(methanediol ethanedioate) + water)
- And another:
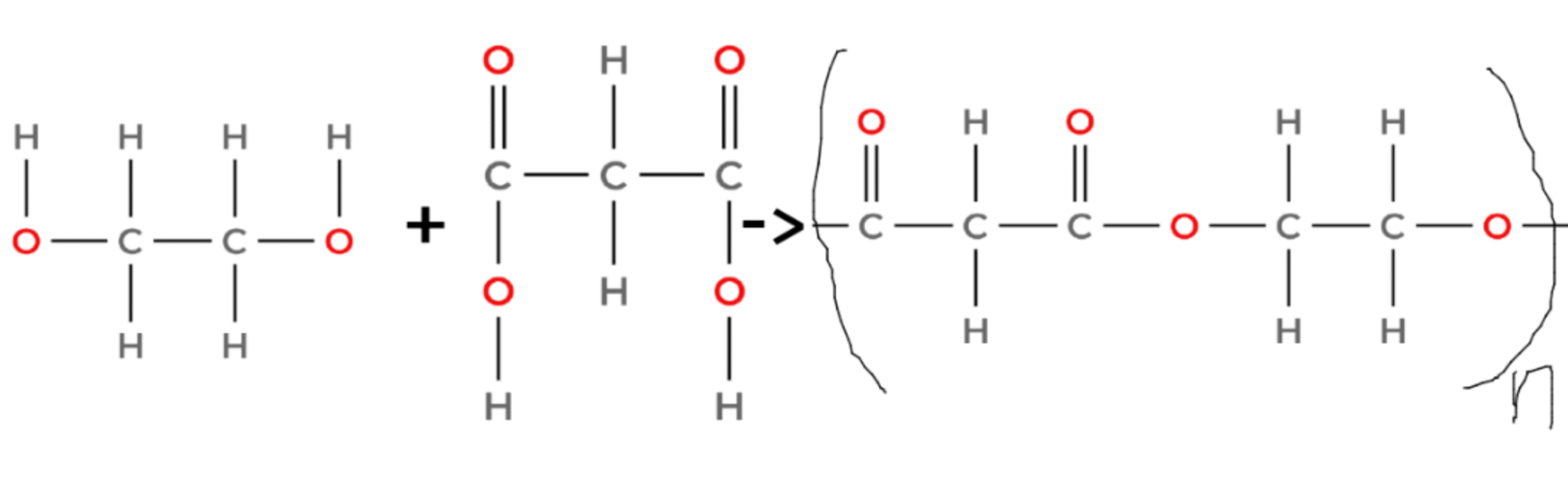 (ethanediol + propanedioic acid → poly(ethanediol propanedioate) + water)
(ethanediol + propanedioic acid → poly(ethanediol propanedioate) + water)
Polyamides
- Polyamides are formed from dicarboxylic acids and diamines (amines with two -NH2 groups).
- The -COOH group of the dicarboxylic acid reacts with the -NH2 group of the diamine to form an amide link (-CONH-) and a molecule of water.
- An example of a polyamide is nylon-6,6, which is made from the dicarboxylic acid
hexanedioic acid and the diamine hexane-1,6-diamine.
- Polyamides are used to make fibres for clothing and carpets.
- Here is how to make an example polyamide:
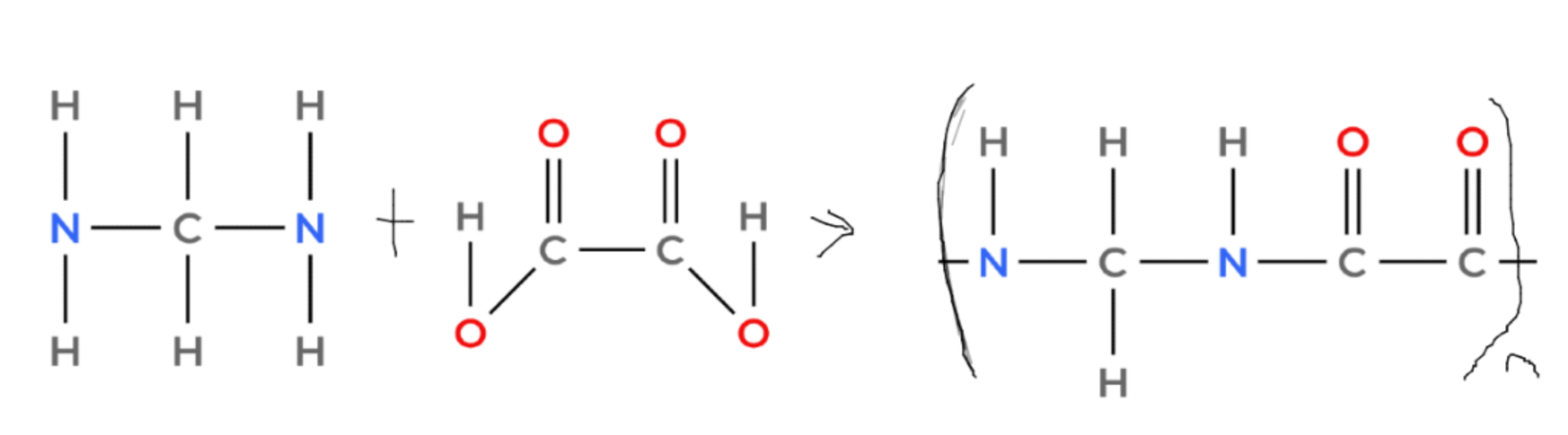 (and H2O)
(and H2O)
7.3.3 Amino acids
(from spec)
- Amino acids have two different functional groups in a molecule.
- Amino acids react by condensation polymerisation to produce
polypeptides.
- For example: glycine is H2NCH2COOH and polymerises to produce the polypeptide:
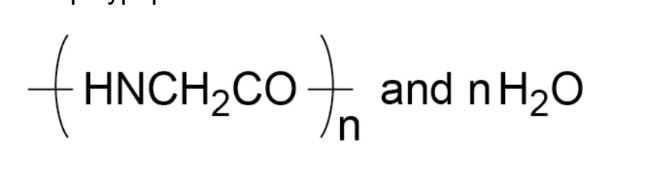 - Different amino acids can be combined in the same chain to produce proteins.
- Different amino acids can be combined in the same chain to produce proteins.
7.3.4 DNA (deoxyribonucleic acid) and other naturally occurring polymer
Carbohydrates
- Glucose and fructose are examples of sugar monomers, called monosaccharides.
- Monosaccharides bond together to produce polymers, called polysaccharides.
- Starch and cellulose are both made from glucose monomers joined by condensation polymerisation.
Proteins
- Amino acids have two different functional groups.
- One group is basic (the amine group -NH2) and one is acidic (the carboxylic acid group -COOH).
- Amino acids react by condensation polymerisation to produce polypeptides.
- E.g. glycine (H2NCH2COOH) polymerises to produce the polypeptide (-HNCH2CO-)n and n H2O.
Polypeptides
- Different amino acids can be combined in the same chain to produce polypeptide chains, which form proteins.
unfinished
DNA
- DNA is a polymer that stores genetic information in cells.
- DNA is made from two polymer chains, made from four different monomers called nucleotides,
arranged in a double helix structure.
- Each nucleotide consists of a sugar, a phosphate group, and a nitrogenous base.
- The sugar and phosphate groups form the backbone of the DNA strand, while the
nitrogenous bases pair up to form the rungs of the DNA ladder.
- The sequence of nitrogenous bases in DNA encodes genetic information.
- Other naturally occurring polymers include carbohydrates (e.g. starch, cellulose; both made from sugar)
and proteins (polypeptides).
