Introduction to organic chemistry (not 7.1, but should come first imo)
yknow this seems like the branch of chem i'll actually enjoy
- Organic chemistry: The study of molecules made from chains of carbon atoms covalently bonded together.
- Hydrocarbon: A compound made up of hydrogen and carbon only.
- Saturated molecule: An organic molecule wtih no carbon-carbon double bonds (e.g. C=C).
- Homologous series: Molecules that have the same functional group
but differ by the addition of a CH2.
- Functional group: The atoms in an organic molecule that react. They determine the chemical
properties of the molecule.
Alkanes
- Name: words (IUPAC [International Union of Pure and Applied Chemistry; you dont need to know that dw])
- e.g. methane, ethane, propane, butane, pentane
- also known as alkanes
- Molecular formula: how many of each atom in the molecule
- e.g. CH4, C2H6
- Empirical formula: Simple whole number ratio
- e.g. CH4, CH3
- Displayed/structural formula: Draw out the structure showing the covalent bonds
- e.g. stick diagram (for C2H6):
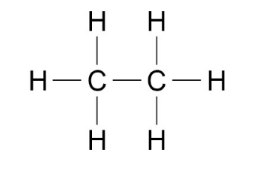
- General formula: all molecules in the same homologous series share a general formula (algebraic)
- The general formula for the homologous series of alkanes is CnH2n+2
Functional groups
- C=C being present means alkene.
- OH being present means alcohol.
- O=C-OH being present means carbonic acid.
- O=C-O being present means ester.
- Alkanes _do not_ have functional groups.
- Alkanes are saturated hydrocarbons, meaning they consist only of carbon (C)
and hydrogen (H) atoms with single bonds between the carbon atoms.
- Despite this, we say the functional group in an alkane is just C-C, but they don't really have one.
- Numbering of aklanes is: Meth-, Eth-, Prop-, But-, Pent-, Hex-, Hept-, Oct-, Non-, Dec-
Alkanes - Functional group C-C
| No. of carbons | Name | Molecular formula | Displayed (structural) formula |
|---|---|---|---|
| 1 | Methane | CH4 | 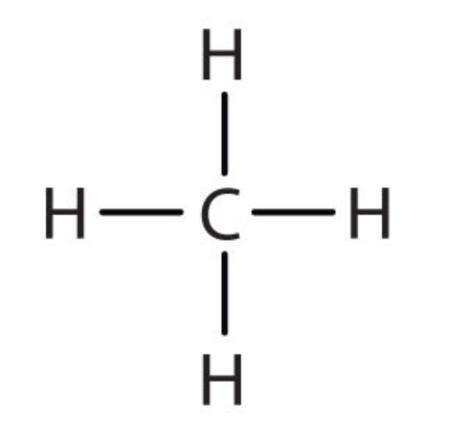 |
| 2 | Ethane | C2H6 | 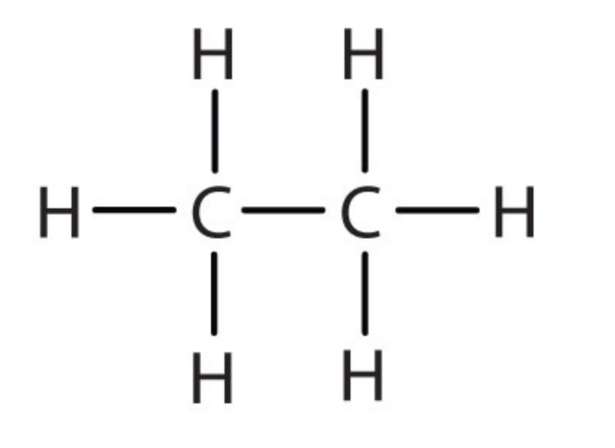 |
| 3 | Propane | C3H8 | 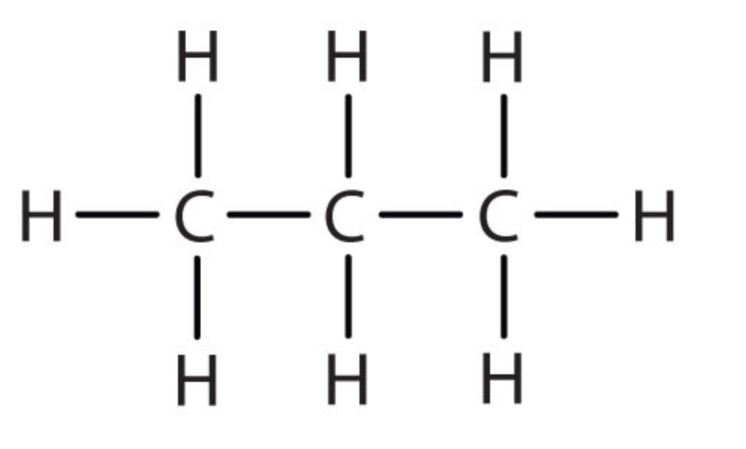 |
| 4 | Butane | C4H10 | 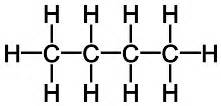 |
Alkenes - Functional group C=C
| No. of carbons | Name | Molecular formula | Displayed (structural) formula |
|---|---|---|---|
| 2 | Ethylene | C2H4 | 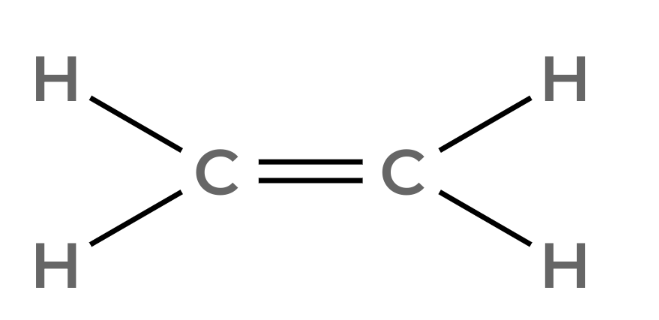 |
| 3 | Propene | C3H6 | 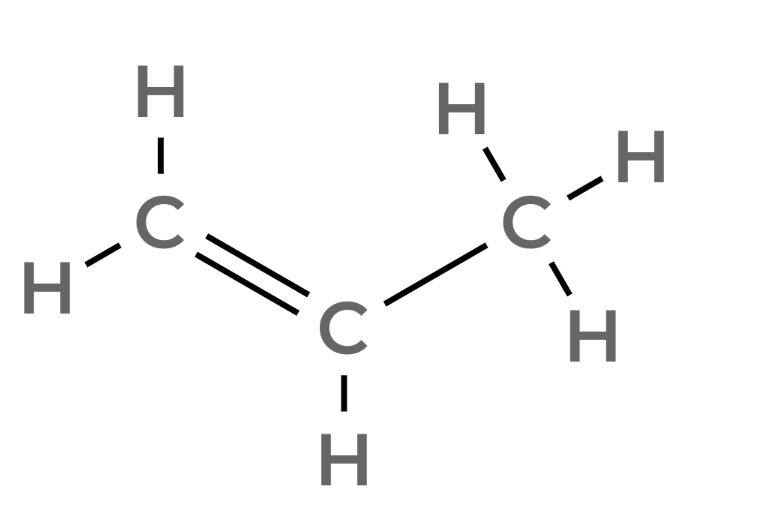 |
| 4 | Butene | C4H8 | 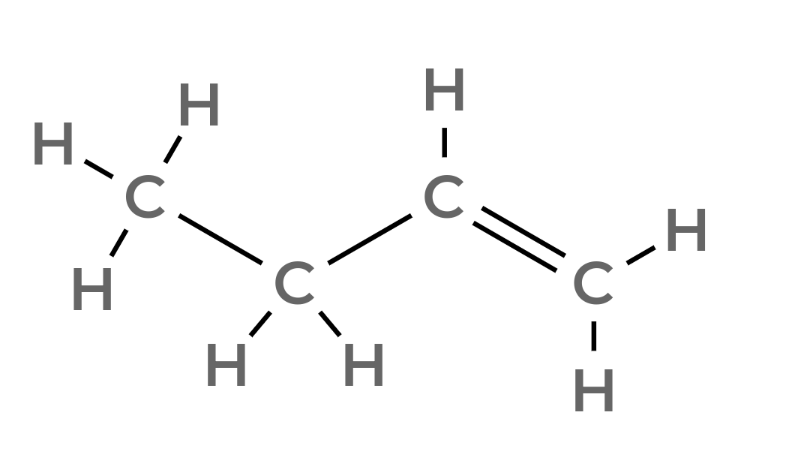 |
Alcohols - Functional Group -OH
| No. of carbons | Name | Molecular formula | Displayed (structural) formula |
|---|---|---|---|
| 1 | Methanol | CH3OH | 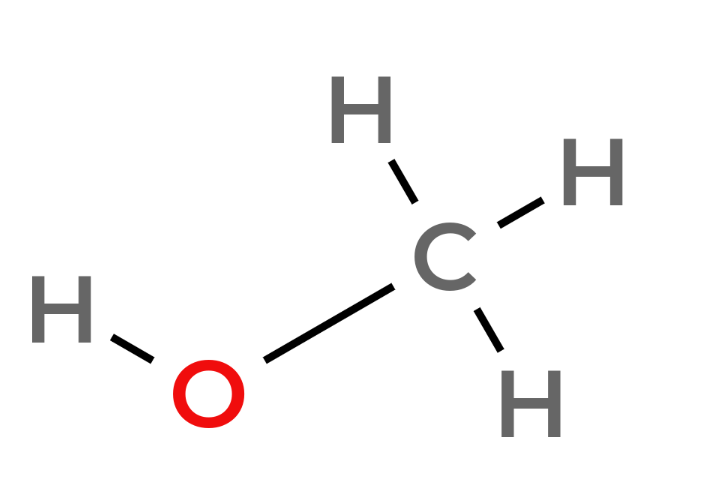 |
| 2 | Ethanol | C2H5OH | 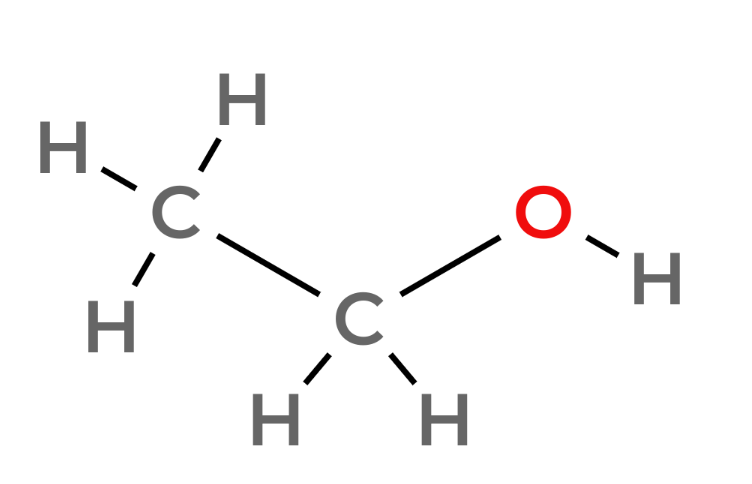 |
| 3 | Propanol | C3H7OH | 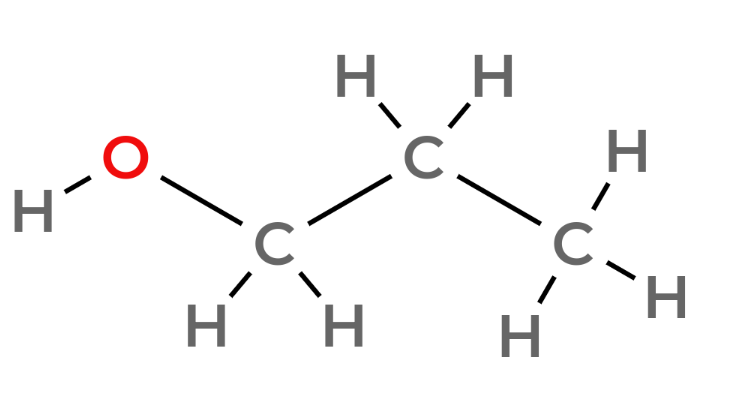 |
| 4 | Butanol | C4H9OH | 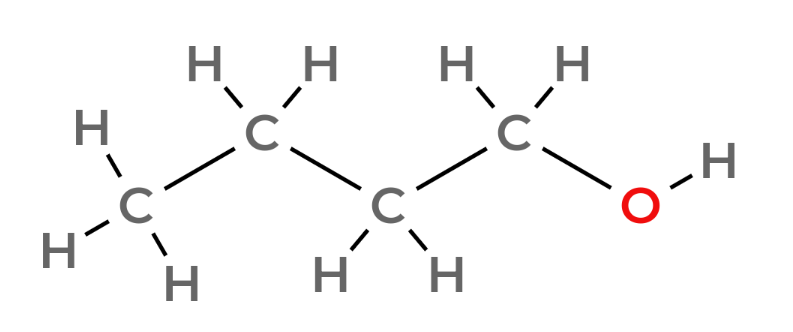 |
notice the pattern - an extra carbon with two hydrogens attached to it per number
Carboxylic acids - Functional Group -COOH
| No. of carbons | Name | Molecular formula | Displayed (structural) formula |
|---|---|---|---|
| 1 | Methanoic Acid | HCOOH | 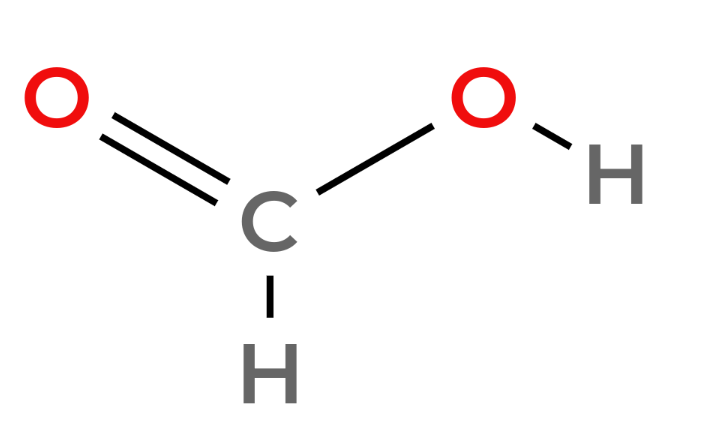 |
| 2 | Ethanoic Acid | CH3COOH | 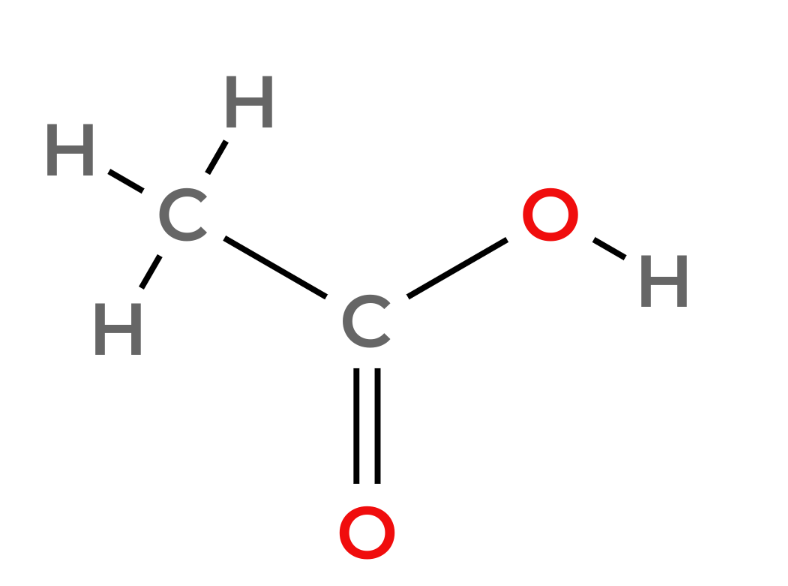 |
| 3 | Propanoic Acid | C2H5COOH | 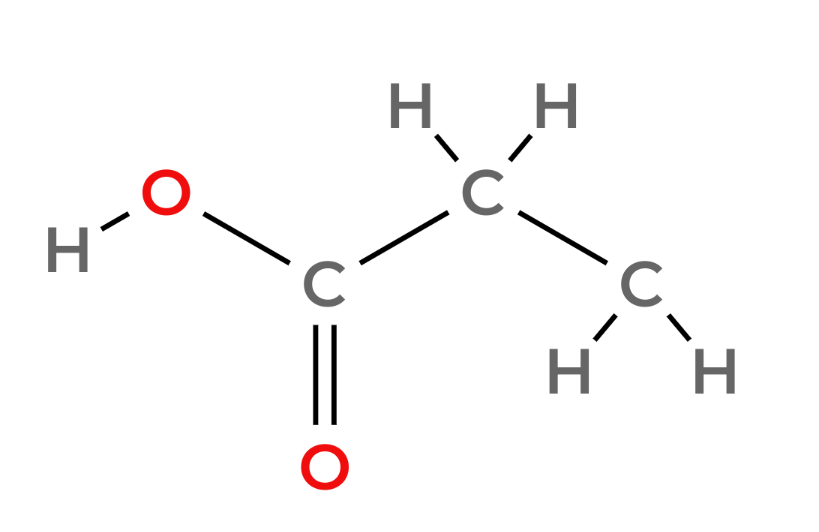 |
| 4 | Butanoic Acid | C3H7COOH | 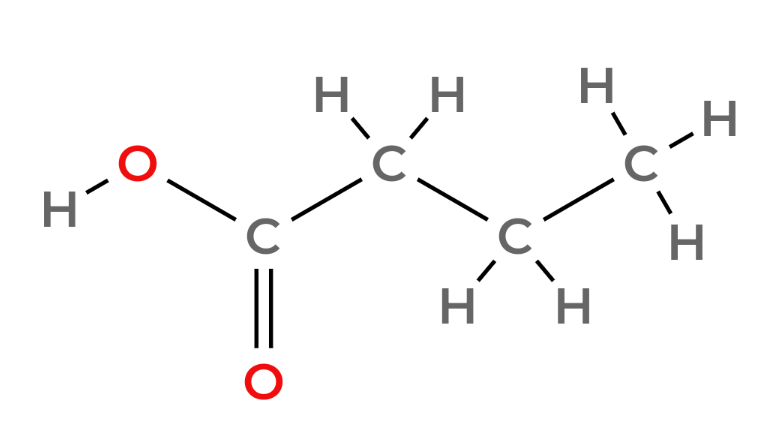 |
