8.1 Purity, formulations and chromatography
8.1.1 Pure Substances
- In chemistry, a pure substance is a single element or compound
not mixed with any other substance.
- In science we would not refer to a substance such as milk as pure because
it is a mixture of a number of different substances. This is different from everyday
language, where a pure substance can mean a substance that has had nothing added to it,
so it is unadulterated and in its natural state, eg pure milk.
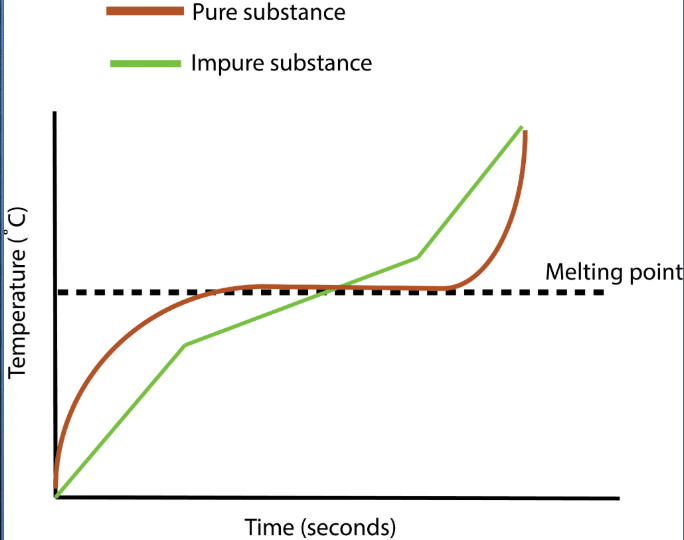
Identifying Pure/Impure Substances
- Pure substances have specific melting and boiling points. These can be
used to distinguish pure substances from impure substances.
| Substance | Melting point (°C) |
|---|---|
| A | 42 |
| B | 104 |
| C | 76-82 |
| D | 35 |
8.1.2 Formulations
- A formulation is a mixture that has been designed as a useful product. Many products are complex mixtures in which each
chemical has a particular purpose
- Formulations are made by mixing the components in carefully measured quantities to ensure
that the product has the required properties.
- Formulations include fuels, cleaning agents, paints, medicines, alloys, fertilisers, and foods.
8.1.3 Chromatography
- Chromatography can be used to separate mixtures and can give information to help identify substances.
- It involves a stationary phase and a mobile phase.
- The stationary phase is the paper
- The mobile phase is the solvent (water)
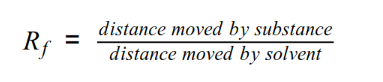 - If a pigment does not travel very fast, it will not travel far up the paper and have a
lower Rf value.
- If a pigment does not travel very fast, it will not travel far up the paper and have a
lower Rf value.- The pigment could have a strong attraction to the stationary phase (with strong intermolecular forces) and/or a weak attraction to the mobile phase.
- If a pigment does travel quite fast, it will travel further up the paper and have a higher Rf value.
- The pigment could have a weak attraction to the stationary phase (with weak intermolecular forces) and/or a strong attraction to the mobile phase.
- This means that different compounds have different Rf values in different solvents, which can be used to help identify the compounds.
Chromatography Experiment
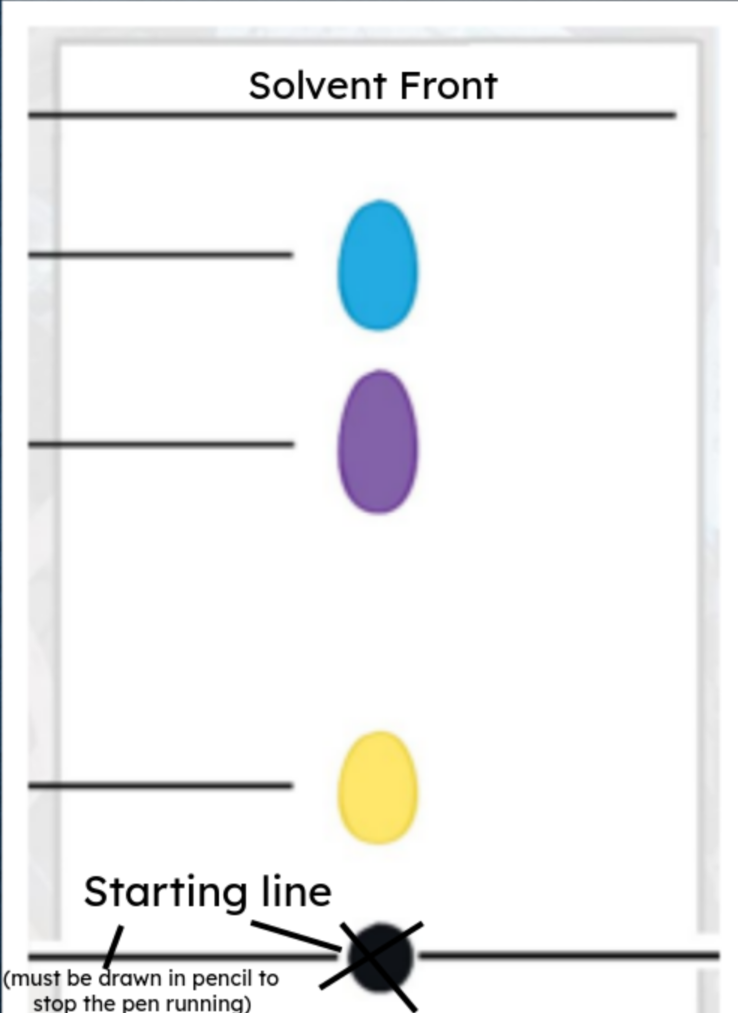 - The black dot is a mixture, because it separates out into multiple colours.
- The black dot is a mixture, because it separates out into multiple colours.
- Yellow is likely to have the strongest attraction to the stationary phase, as it travelled the least.
- Blue is likely to have the strongest attraction to the mobile phase, as it travelled the most.
Rf Calculation
- The solvent front is 10.5cm from the starting line.
- Yellow is 2cm from the starting line.
- Purple is 6.5cm from the starting line.
- Blue is 9cm from the starting line.
- Using the Rf equation:
- Yellow: Rf = 2/10.5 = 0.19
- Purple: Rf = 6.5/10.5 = 0.62
- Blue: Rf = 9/10.5 = 0.86
